Benzaldehyde: Properties, Reactions, Production and Uses

Benzaldehyde is the most important aromatic aldehyde, both in nature and industry, with the formula C7H6O. It is a colorless liquid with a distinctive odor resembling bitter almond.
Benzaldehyde is found in many plants, both bound and unbound. One important natural source of benzaldehyde is amygdalin, a glycoside found in bitter almonds.
The distinctive almond fragrance is due to small amounts of free benzaldehyde, which is produced when amygdalin is hydrolyzed. Benzaldehyde is also the main component of the essential oils extracted from the kernels of peaches, cherries, and apricots.
In 1818 and 1819, VOGEL and MATRÈS independently reported the extraction of a volatile oil from bitter almonds, along with hydrocyanic acid. In 1832, WÖHLER and LIEBIG conducted a comprehensive study of this oil and determined its chemical composition and relationship to benzoic acid and benzoyl chloride.
Table of Contents
1. Physical Properties of Benzaldehyde
Benzaldehyde is a colorless, highly refractive liquid that is volatile in the presence of steam. It is miscible with numerous organic solvents and can be mixed with concentrated sulfuric acid, liquid carbon dioxide, liquid ammonia, methylamine, and diethylamine at 25 °C.
Here is a summary of the physical properties of benzaldehyde:
| Property | Value |
|---|---|
| Molecular weight | 106.13 g/mol |
| Color | Colorless |
| Odor | Bitter almonds |
| Physical state | Liquid |
| Boiling point | 179 °C at 101.3 kPa |
| Melting point | -56 °C |
| Refractive index | 1.5450 |
| Density | 1.063 g/cm³ at 0 °C, 1.046 g/cm³ at 20 °C, 1.018 g/cm³ at 50 °C |
| Specific heat capacity | 1.676 J g⁻¹ K⁻¹ at 25 °C |
| Heat of evaporation | 371.0 J/g at 179 °C |
| Standard heat of combustion | 33.19 kJ/g |
| Flash point | 64.5 °C |
| Autoignition temperature | 190 °C |
| Lower explosive limit | 1.4 vol% |
| Dynamic viscosity (η) | 1.40 x 10⁻³ Pa · s at 25 °C
1.11 x 10⁻³ Pa · s at 40 °C |
| Surface tension (s) | 40.04 x 10⁻³ N/m at 20 °C |
| Dipole moment (m) | 2.92 D (9.74 x 10⁻³⁰ C m) in liquid benzene |
| Dielectric constant (εr) | 17.7 at 25 °C |
2. Chemical Reactions of Benzaldehyde
Benzaldehyde and its derivatives have similar chemical behavior to aliphatic aldehydes, but their reactivity is reduced due to the resonance of the p electrons from the carbonyl group with the aromatic ring.
This unique property allows benzaldehyde to form various compounds, such as Schiff bases with amines, oximes with hydroxylamine, hydrazones with phenylhydrazine, and acetals with alcohols. It also reacts with hydrogen cyanide, sodium bisulfite, and Grignard compounds.
One notable synthesis of benzaldehyde is the Strecker reaction, which uses ammonia and hydrogen cyanide to form an aminonitrile intermediate that can be saponified to yield DL-2-phenylglycine.
Benzaldehyde can also autoxidize in air to form benzoic acid. This process is affected by light and can be accelerated by peroxides or heavy-metal salts, but slowed down by antioxidants such as phenolic compounds and diphenylamine. Benzaldehyde can also be oxidized to benzoic acid by agents such as nitric acid and chromium(VI) oxide.
Reducing or hydrogenating benzaldehyde under different conditions results in various products, including benzyl alcohol, dibenzyl ether, benzoin, 1,2-diphenylethane-1,2-diol, stilbene, toluene, and methylcyclohexane. Catalytic hydrogenation, which is used industrially, produces benzyl alcohol.
Reduction with aluminum alcoholates (Meerwein-Ponndorf-Verley reduction) is another method for obtaining benzyl alcohol, and this process can also reduce unsaturated aldehydes, like cinnamaldehyde, while retaining olefinic double bonds.
Benzaldehyde reacts with ammonia and hydrogen in the presence of hydrogenation catalysts to produce benzylamine, which has significant industrial applications. Side-chain chlorination of benzaldehyde results in the formation of benzoyl chloride.
Like aliphatic aldehydes, benzaldehyde also participates in condensation reactions with various organic compounds containing active hydrogen atoms. Some of these reactions are used in industry. For example, the Claisen-Schmidt condensation with acetaldehyde and aqueous alkali produces cinnamaldehyde.

The Perkin condensation with acetic anhydride in the presence of specific condensing agents leads to the industrial production of cinnamic acid.

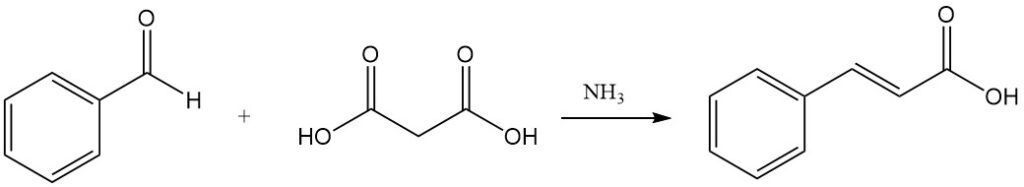
Knoevenagel condensation with malonic acid, catalyzed by weakly basic substances like ammonia and amines, is another route to cinnamic acid.
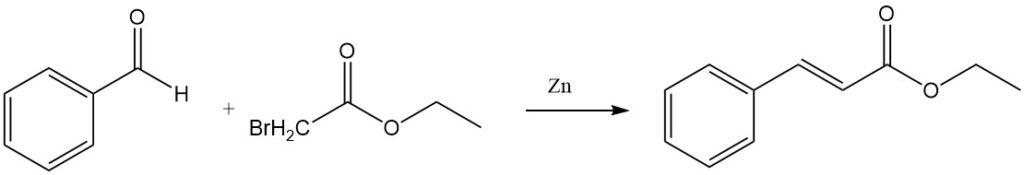
The Reformatsky reaction of benzaldehyde and ethyl bromoacetate in the presence of activated zinc yields ethyl cinnamate.
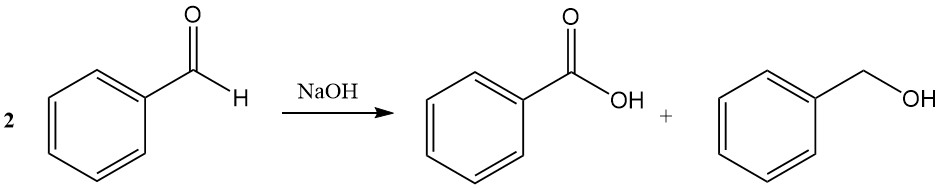
The Cannizzaro reaction of benzaldehyde leads to the formation of benzoic acid and benzyl alcohol in the presence of concentrated sodium hydroxide or potassium hydroxide.
The Claisen-Tishchenko condensation of benzaldehyde is catalyzed by sodium and aluminum benzylate to form benzyl benzoate.
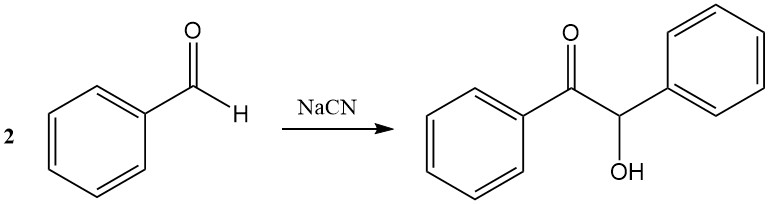
In the benzoin condensation, two benzaldehyde molecules combine in the presence of cyanide to form benzoin. Thiazolium salts can replace cyanide as catalysts.
Benzaldehyde reacts differently with ammonia than aliphatic aldehydes, the reaction continues until the formation of 1-phenyl-N,N’-bis(phenylmethylene)-methanedi-amine (hydrobenzamide).

Benzaldehyde treated with Fehling’s solution forms benzyl alcohol and benzoic acid, but no copper(I) oxide. Aromatic aldehydes do not polymerize or form cyclic compounds like aliphatic aldehydes.
Benzaldehyde condenses with phenols, aromatic amines, and benzene to form triphenylmethane derivatives. This reaction is used industrially to make Malachite Green dyes.
The electrophilic substitution of the aromatic nucleus in benzaldehyde and other aromatic aldehydes, such as chlorination, nitration, and sulfonation, primarily occurs in the meta position.
3. Production of Benzaldehyde
Benzaldehyde is mainly produced by hydrolyzing benzal chloride or partially oxidizing toluene. Other manufacturing processes exist, but they are not currently industrially important.
3.1. Hydrolysis of Benzal Chloride
Benzaldehyde is produced by hydrolyzing benzal chloride, a compound easily obtained by chlorinating toluene. This hydrolysis can be done in alkaline or acidic conditions.

Alkaline hydrolysis:
Benzal chloride can be saponified with various alkaline agents, such as calcium hydroxide, calcium carbonate, sodium hydrogencarbonate, or sodium carbonate. Sodium carbonate is the preferred agent because it minimizes side reactions.
In an older process, benzal chloride is saponified with a slight excess of 15% sodium carbonate solution at 138 °C. The reactor is made of specific materials, such as a carbon steel agitator vessel lined with Heresite (a phenolic resin) and a copper-silicon alloy agitator.
This process minimizes the chlorine content of the distilled product to less than 0.01%. A mixture of benzal chloride and benzotrichloride, commonly obtained in the side-chain chlorination of toluene, can also be hydrolyzed. In this process, benzotrichloride is converted to sodium benzoate and subsequently to benzoic acid.
In a more recent continuous process, benzal chloride and the alkaline saponifying agent are reacted in a flow reactor using an unreactive organic solvent. The extracted benzaldehyde is then separated from the aqueous alkaline phase by flowing an unreactive organic solvent in the opposite direction. This continuous process integrates the reactor, extraction zone, and washing zone in a single apparatus.
Alternatively, benzal chloride can be converted into benzaldehyde by boiling it with an aqueous solution of hexamethylenetetramine. The Sommelet reaction can also be used to produce benzaldehyde from industrial mixtures of benzyl chloride and benzal chloride.
Acid hydrolysis:
Benzal chloride can also be hydrolyzed in acidic conditions, using acids and metal salts as catalysts. This yields high yields of benzaldehyde (more than 90%) and hydrogen chloride, which can be recovered as concentrated hydrochloric acid.
In the past, this hydrolysis was often performed in the presence of concentrated sulfuric acid, resulting in the formation of large amounts of dilute sulfuric acid as waste. This method is suitable for the hydrolysis of certain substituted benzaldehydes that are difficult to saponify through other means.
Metal salts, particularly iron or zinc salts, can also catalyze the hydrolysis of benzal chloride. It is important to prevent the accumulation of water in the reaction mixture, as it can reduce the catalyst’s activity.
The process can be carried out using various catalysts, such as zinc phosphate, zinc laurate, tin chlorides, copper(II) chloride, and more. The reaction can be performed batchwise or continuously in a cascade of reactors.
Vapor-phase hydrolysis:
An innovative continuous process involves the vapor-phase hydrolysis of benzal chloride at elevated temperatures, catalyzed by activated carbon treated with acid or impregnated with metal chloride or sulfate. This process yields a high benzaldehyde yield of 97%.
This method is particularly suitable for hydrolyzing trifluoromethyl-substituted benzal chlorides, which are otherwise challenging to convert into the corresponding benzaldehydes. A similar vapor-phase process involves hydrolyzing benzal chloride to benzaldehyde at 300 °C using a catalyst like silicon dioxide or aluminum oxide.
3.2. Oxidation of Toluene
Benzaldehyde can be produced from toluene by partial oxidation, which can be done in either the gas phase or liquid phase. The conditions must be carefully controlled to favor partial oxidation, as benzaldehyde can be further oxidized to benzoic acid and other products.

Gas-phase oxidation
In the gas-phase process, toluene vapor and oxygen are passed through a catalyst bed at high temperatures (250-650 °C). The reaction is very hot, so it is important to cool it effectively.
To improve the yield of benzaldehyde, it is helpful to dilute the toluene vapor and oxygen with an inert gas, such as water vapor, nitrogen, or carbon dioxide.
The best conditions include low conversion rates (10-20% per pass), short residence times (0.1-1.0 seconds), and precise control of the oxygen amount. Even under these conditions, the yield is typically only 40-60% of the theoretical yield based on toluene.
This gas-phase oxidation of toluene also produces other compounds, such as maleic anhydride, citraconic anhydride, phthalic anhydride, anthraquinone, cresol, acetic acid, and significant amounts of benzoic acid, carbon monoxide, and carbon dioxide. Adding potassium sulfate or sodium fluoride to the catalyst or using high pressure can reduce complete combustion.
Various oxide catalysts, typically containing molybdenum and other elements such as iron, nickel, cobalt, antimony, bismuth, vanadium, phosphorus, samarium, tantalum, tin, and chromium, are often used for gas-phase oxidation.
Some catalysts combine palladium and phosphoric acid on activated carbon, while others consist of mixed oxides of silver and transition metals. Another process uses a mixed-oxide catalyst with uranium, copper, iron, phosphorus, tellurium, and lead in addition to molybdenum.
Liquid-phase oxidation
Liquid-phase oxidation of toluene with oxygen is also a common method. This is usually done in the presence of catalysts such as cobalt, nickel, manganese, iron, or chromium compounds. Lead compounds, ruthenium compounds, thallium salts of organic acids, and various promoters have also been used, but they can lead to corrosion.
Benzaldehyde formation in the liquid phase may also involve oxidation with other agents such as methanol, acetaldehyde, benzoic acid, acetic acid, or the addition of water.
Distillation is commonly used to purify the crude benzaldehyde obtained from these processes, with further purification steps if necessary to remove impurities that discolor the product.
Byproduct benzaldehyde
In large-scale processes for phenol and caprolactam production, significant amounts of benzaldehyde are often produced as by-products. As a result, dedicated oxidation processes for exclusive benzaldehyde production are less commonly used in industrial settings.
These byproduct benzaldehyde streams are typically processed to obtain pure benzaldehyde. It is important to note that even when producing benzaldehyde as the primary product by catalytic liquid-phase oxidation, significant amounts of benzoic acid and other byproducts may be formed.
The crude benzaldehyde is usually refined by distillation at reduced pressure in a stainless steel column.
Other oxidation processes
Processes that involve oxidizing toluene with agents such as manganese dioxide in sulfuric acid, sodium persulfate, chromium(VI) oxide in acetic anhydride, or chromyl chloride are generally not of industrial importance due to waste water disposal issues.
3.3. Other Production Processes
There are other ways to make benzaldehyde, but they are not as widely used as the more common methods.
- Benzene and carbon monoxide reaction: Benzaldehyde can be made by reacting benzene with carbon monoxide. However, this method is not commonly used in industry.
- Benzyl alcohol oxidation or dehydrogenation: Benzaldehyde can also be made by oxidizing or dehydrogenating benzyl alcohol. This method is possible, but it is not the main way to make benzaldehyde on a large scale.
- Ruthenium-catalyzed oxidation of styrene with periodate or hypochlorite: Benzaldehyde can be made by using ruthenium to catalyze the oxidation of styrene with periodate or hypochlorite. This method is not commonly used in industry to make benzaldehyde.
- Hydrolysis of benzyl chloride and benzal chloride mixtures: Benzaldehyde can be made by hydrolyzing mixtures of benzyl chloride and benzal chloride in dilute nitric acid, using vanadium pentoxide as a catalyst. However, this is not the main way to make benzaldehyde in industry.
- Reduction of benzoyl chloride or methyl benzoate: Benzaldehyde can also be made by reducing benzoyl chloride or methyl benzoate. These methods are generally not of industrial importance for the production of benzaldehyde and are more likely to be used in the synthesis of specific nuclear-substituted derivatives.
In summary, these other methods for benzaldehyde production are not commonly used in industry. The methods described earlier are the primary approaches for its large-scale manufacture.
4. Uses of Benzaldehyde
Benzaldehyde is a versatile and important chemical with a wide range of applications. It is used to produce a variety of odorants and flavors, including those found in natural bitter almond oil, perfumes, soaps, foods, and drinks.
Benzaldehyde is used to produce derivatives that are used in the perfume and flavor industries, such as cinnamaldehyde, cinnamyl alcohol, cinnamic acid, and benzyl benzoate.
Benzaldehyde is also used to produce triphenylmethane dyes, such as the leuco base of Malachite Green and the acridine dye benzoflavin.
In the pharmaceutical industry, benzaldehyde is used as an intermediate in the manufacture of chloramphenicol, ephedrin, ampicillin, diphenylhydantoin, and other products.
Other important chemical intermediates obtained from benzaldehyde include benzoin, benzylamine, benzyl alcohol, mandelic acid, and 4-phenyl-3-buten-2-one (benzylideneacetone).
Benzaldehyde is also used in photochemistry, as a corrosion inhibitor and dyeing auxiliary, in the electroplating industry, and in the production of agricultural chemicals.
5. Toxicology of Benzaldehyde
Benzaldehyde is used in food, cosmetics, pharmaceuticals, and soap as a flavoring and fragrance agent. It is generally safe for these uses. Benzaldehyde also has industrial and agricultural applications.
Benzaldehyde has moderate acute toxicity. The oral LD50 (dose that kills 50% of a test population) is 1.3 g/kg in rats and 1 g/kg in guinea pigs. The estimated probable lethal dose for a 70-kg human is 50 mL. Subchronic oral administration to rodents showed no adverse effects at daily doses of 400 mg/kg in rats and 300-600 mg/kg in mice.
However, higher doses caused damage to the brain, kidney, and forestomach. Mutagenicity tests were negative. There are no reported studies on the carcinogenic, teratogenic, or reproductive effects of benzaldehyde.
Toxic effects of benzaldehyde include depression, inactivity, tremors, seizures, and coma. Fatality can result from respiratory depression. Benzaldehyde has a weak local anesthetic effect and is mildly irritating to the eye and upper respiratory tract. Skin irritation is moderate, and some individuals may develop an allergic reaction.
To minimize risks, avoid contact with benzaldehyde. If contact is necessary, wear gloves and protective clothing. In poorly ventilated areas, use self-contained breathing apparatus to prevent inhalation exposure.
Reference
- Benzaldehyde; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_463.pub2
