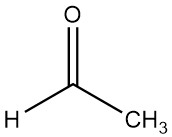
Acetaldehyde, also known as ethanal, is an organic chemical compound with the formula CH3CHO. It is a volatile, low-boiling, and highly flammable liquid characterized by its strong odor.
Acetaldehyde was first observed by SCHEELE in 1774 when black manganese dioxide and sulfuric acid reacted with alcohol. Its composition was later explained by LIEBIG in 1835, who obtained pure acetaldehyde by oxidizing ethanol with chromic acid.
Due to its remarkable chemical reactivity, acetaldehyde plays a important role as an intermediate in the manufacturing of various compounds, including acetic acid, acetic anhydride, ethyl acetate, peracetic acid, butanol, 2-ethylhexanol, pentaerythritol, chlorinated acetaldehydes (chloral), glyoxal, alkyl amines, pyridines, and other chemicals.
Its initial commercial application involved the production of acetone through acetic acid synthesis, which took place between 1914 and 1918 in Germany (Wacker-Chemie and Hoechst) as well as in Canada (Shawinigan).
Acetaldehyde is also present in the metabolic processes of plants and animals, albeit in small quantities. Higher levels of acetaldehyde can disrupt biological processes.
Acetaldehyde is naturally found in trace amounts during alcoholic fermentation, contributing to the flavor of beverages like beer, wine, and spirits. Additionally, it has been detected in plant juices, essential oils, roasted coffee, and tobacco smoke.
Various commercial methods are employed to produce acetaldehyde. These include dehydrogenation or oxidation of ethanol, water addition to acetylene, partial oxidation of hydrocarbons, and direct oxidation of ethylene.
Table of Contents
2. Physical Properties of Acetaldehyde
Acetaldehyde, with the molecular formula C2H4O and a molar mass of 44.054 g/mol, is a transparent liquid characterized by a strong and suffocating odor. When diluted, it gives off a slightly fruity scent.
Acetaldehyde is fully soluble in water and most organic solvents. It does not form any azeotropes with water, methanol, ethanol, acetone, acetic acid, or benzene.
However, it does form binary azeotropes with butane (boiling point -7 °C, containing 84% by weight of butane) and diethyl ether (boiling point 18.9 °C, containing 23.5% by weight of ether).
Some physical properties of acetaldehyde are listed below:
- Boiling point at 101.3 kPa = 20.16 °C
- Melting point = -123.5 °C
- Critical temperature = 181.5 °C
- Relative density (t) = 0,8045-0,001325.t (t in °C)
- Refractive index (t) = 1,34240-0,0005635.t (t in °C)
- Viscosity of liquid at 20 °C = 0.21 mPa.s
3. Chemical Reactions and Uses of Acetaldehyde
Acetaldehyde is a remarkably reactive compound that exhibits all characteristic reactions of aldehydes, as well as those associated with an alkyl group in which hydrogen atoms are activated by the presence of a carbonyl group in the α position.
When heaated to temperatures exceeding 420 °C, acetaldehyde undergoes decomposition, resulting in the formation of methane and carbon monoxide.
3.1. Addition Reactions
Acetaldehyde readily reacts with water, forming an unstable hydrate. However, solid isolable hydrates are only known to exist with chlorinated acetaldehydes. Alcohols can add to acetaldehyde, producing hemiacetals, which, in the presence of acids and subsequent removal of water, can form acetals when combined with additional alcohol.
Diols lead to the formation of cyclic acetals. For example, ethylene glycol and acetaldehyde yield 2-methyl-1,3-dioxolane, while 1,3-propanediol and acetaldehyde produce 2-methyl-1,3-dioxane.

The reaction between acetaldehyde and aqueous sodium bisulfite solution yields a crystalline adduct, from which acetaldehyde can be liberated.
Dry ammonia reacts with acetaldehyde, resulting in the formation of crystalline acetaldehyde ammonia.
Acetaldehyde and hydrocyanic acid undergo a reaction to produce lactonitrile (α-hydroxypropionitrile), which is a potential intermediate in the production of acrylonitrile.
The reaction between acetaldehyde and acetic anhydride yields ethylidene diacetate, an intermediate in the vinyl acetate process carried out by Celanese Corp.
3.2. Derivatives of Aldol Addition
Under the presence of alkaline catalysts or with mild heating, two molecules of acetaldehyde combine to form acetaldol. At higher temperatures, water is easily eliminated from acetaldol, resulting in the formation of crotonaldehyde. However, the industrial significance of further condensation to form aldehyde resins, such as synthetic shellac, has diminished.
Urea and acetaldehyde can condense in the presence of H2SO4, leading to the formation of crotonylidenediurea (6-methyl-4-ureidohexahydropyrimidin-2-one), which is utilized as a long-term nitrogen fertilizer.

Acetaldehyde serves as an intermediate in the synthesis of butadiene. It begins with acetylene and proceeds through acetaldol and its hydrogenation product, 1,3-butanediol. This process was introduced in the early 20th century and is still practiced on a commercial scale in certain Eastern European countries.
Acrolein is obtained through the aldol condensation of acetaldehyde and formaldehyde, followed by water elimination, similar to the formation of crotonaldehyde. However, this method lacks contemporary commercial significance. Conversely, the production of pentaerythritol from acetaldehyde and a fourfold amount of formaldehyde in the presence of Ca(OH)2 or NaOH holds significant industrial importance.
3.3. Reaction with Nitrogen Compounds
Primary amines react with acetaldehyde to form Schiff bases, represented as CH3CH=NR. Nitrogen compounds like hydroxylamine, hydrazine, phenylhydrazine, and semicarbazide can react with acetaldehyde, yielding easily crystallizable compounds used for analytical determination and characterization of aldehydes. Examples include semicarbazone (mp 162 – 163 °C), p-nitrophenylhydrazone (mp 128.5 °C), 2,4-dinitrophenylhydrazone (mp 168 °C), and oxime (mp 47 °C).
Similar methods can be employed for the characterization of other aldehydes and ketones, as their analogous derivatives generally possess distinct and well-defined melting points.
The synthesis of pyridine and pyridine derivatives has gained increasing importance. 5-Ethyl-2-methylpyridine can be obtained by reacting aqueous ammonia with acetaldehyde in the presence of fluoride ions. Paraldehyde, which gradually releases the monomer, can also be used. A mixure of pyridine and alkylpyridines forms in the presence of formaldehyde or acrolein.

3.4. Oxidation
A significant portion of commercially produced acetaldehyde is employed in the manufacturing of acetic acid through oxidation with oxygen or air.
Acetaldehyde monoperacetate is an intermediate product that decomposes into peracetic acid and acetaldehyde under elevated temperatures and the presence of catalytic amounts of iron or cobalt salts. In the presence of Mn2+ salts, acetic acid can be obtained from acetaldehyde monoperacetate. Moreover, acetic anhydride can be formed by employing Co2+ and Cu2+ salts.

Oxidation with nitric acid results in the formation of glyoxal, while halogenated acetaldehydes can be prepared through halogenation.
Mono-, di-, and trichloroacetaldehyde, as well as tribromoacetaldehyde (bromal), find utility in the production of insecticides (e.g., DDT, DDD), pharmaceuticals, and dyes.
3.5. Reduction
Acetaldehyde can be readily hydrogenated to yield ethanol. Prior to 1939, when petrochemically produced ethylene became available in Europe, this reaction was industrially employed to produce ethanol from acetaldehyde and, consequently, from acetylene.
Mono-, di-, and triethylamine can be synthesized from acetaldehyde, ammonia, and hydrogen in the presence of a hydrogenation catalyst. For further information, refer to the production of aliphatic amines.
3.6. Miscellaneous Reactions
The Tishchenko reaction of acetaldehyde, catalyzed by aluminum alcoholate, results in the production of the commercially important solvent ethyl acetate.
Acetaldehyde serves as a “radical trapping agent” in the polymerization of vinyl compounds, controlling the chain length of the polymers.
3.7. Polymers of Acetaldehyde
3.7.1. Paraldehyde
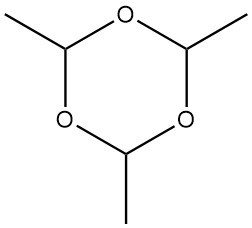
Paraldehyde, 2,4,6-trimethyl-1,3,5-trioxane with a molecular weight of 132.161, is a cyclic trimer of acetaldehyde. It possesses a colorless appearance and emits an ethereal, penetrating odor.
Paraldehyde can be synthesized from acetaldehyde in the presence of acid catalysts, such as sulfuric acid, phosphoric acid, hydrochloric acid, or acid cation exchangers. In a homogeneous reaction, acetaldehyde is added to paraldehyde containing a small amount of sulfuric acid with stirring and cooling.
After completion, stirring continues to establish equilibrium, and the sulfuric acid is neutralized precisely using a sodium salt like sodium acetate, sodium carbonate, or sodium bicarbonate. Fractional distillation separates the reaction mixture into acetaldehyde, water, and paraldehyde.
For continuous production, liquid acetaldehyde at temperatures of 15 – 20 °C or acetaldehyde vapor at temperatures of 40 – 50 °C is passed over an acid cation exchanger, achieving a conversion rate exceeding 90%. Distillation is then employed to separate acetaldehyde from paraldehyde.
Depolymerization involves slow distillation of acetaldehyde in the presence of acid catalysts. Alternatively, paraldehyde can be decomposed in the gas phase using catalysts like HCl, HBr, H3PO4, or cation exchangers. The depolymerization reaction follows a first-order rate.
Paraldehyde finds applications in chemical synthesis as a source of acetaldehyde, eliminating resin formation and other secondary reactions. This synthesis is utilized in the production of pyridines and the chlorination of chloral.
Between 1939 and 1945, paraldehyde was utilized as a motor fuel.
3.7.2. Metaldehyde

Metaldehyde is the cyclic tetramer of acetaldehyde. It forms tetragonal prisms with a melting point of 246.2 °C (closed capillary) and a sublimation temperature of 115 °C. The heat of combustion at constant volume is 3370 kJ/mol.
Metaldehyde is insoluble in water, acetone, acetic acid, and carbon disulfide.
Depolymerization of metaldehyde to acetaldehyde commences at 80 °C and is complete above 200 °C. Acid catalysts like dilute H2SO4 or H3PO4 are used in the depolymerization process.
Metaldehyde does not exhibit the typical reactivity of acetaldehyde. It is stabilized by ammonium carbonate or other weakly basic compounds that neutralize potential acidic catalysts.
Metaldehyde is obtained, alongside significant amounts of paraldehyde, during the polymerization of acetaldehyde in the presence of HBr and alkaline earth metal bromides like CaBr2 at temperatures below 0 °C.
However, the yields are rarely above 8%. Higher yields of 14 – 20% have been reported when 7 – 15% of an aliphatic or cyclic ether is present at temperatures ranging from 0 to 20 °C. Insoluble metaldehyde is subsequently filtered.
Acetaldehyde is then distilled from the filtrate after depolymerization of the paraldehyde and can be recycled back into the polymerization process. However, this recycling of large amounts of acetaldehyde leads to increased process costs due to losses.
Metaldehyde in pellet form is commercially available as a dry fuel (Meta). When combined with bait, metaldehyde is used as a molluscicide.
3.7.3. Polyacetaldehyde
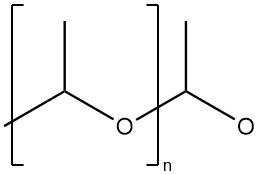
Polyacetaldehyde is a high-molecular-mass polymer with an acetal structure (polyoxymethylene structure).
By using cationic initiators, mainly an amorphous polymer is obtained. Temperatures below -40 °C are preferred in this case. Above -30 °C, mainly paraldehyde and metaldehyde are produced.
The initiator activity also depends on the solvent used. Suitable initiators include H3PO4 in ether and pentane, as well as HCl, HNO3, CF3COOH, AlCl3 in ether, and particularly BF3 in liquid ethylene. Al2O3 and SiO2 also seem to be good initiators.
The polymer has a rubber-like consistency and is soluble in common organic solvents. It depolymerizes at room temperature, liberating acetaldehyde. It evaporates completely within a few days or weeks. Acidic compounds accelerate depolymerization, and amines (e.g., pyridine) stabilize polyacetaldehyde to a certain extent.
3.8. Consumption
In the United States, starting from 1993, acetaldehyde ceased to be employed in the manufacturing processes of acetic acid, butanol, and 2-ethylhexanol, as alternative methods are now employed for their production.
However, the demand for acetaldehyde has increased for other substances such as peracetic acid and pyridine bases.
Consumption of acetaldehyde (t) in 2003
| Products | Consumption (t) |
|---|---|
| Acetic acid/acetic anhydride | 147,000 |
| Acetate esters | 321,000 |
| Pentaerythritol | 80,000 |
| Pyridine and pyridine bases | 83,000 |
| Peracetic acid | 23,000 |
| 1,3-Butylene glycol | 14,000 |
| Others | 98,000 |
Reference
- Acetaldehyde; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_031.pub2


