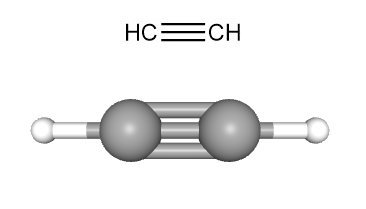
Acetylene also known as ethyne, is a colorless, flammable gas with the chemical formula C2H2. It is the simplest hydrocarbon with a triple bond. It finds widespread use as a fuel in oxy-acetylene welding and cutting due to its incredibly hot flame, and as a chemical building block for various organic chemicals and plastics.
Acetylene was the primary building block for industrial organic chemistry before the widespread adoption of oil as the main feedstock. The calcium carbide process exclusively dominated acetylene production until the 1940s, when thermal cracking methods utilizing methane and other hydrocarbons emerged.
Initial implementations employed electric arcs, but the 1950s witnessed the development of partial oxidation and regenerative processes.
Despite promising initiatives like BASF’s submerged flame process, Hoechst’s HTP, and Hüls’ plasma process, the trend towards ethylene as a foundational chemical remained unwavering.
All acetylene processes, including carbide methods, are energy-intensive, requiring significant heat generation and transfer. They primarily differ in how this energy is generated and applied.
- For a complete article about the industrial production of acetylene, visit the following link.
- For detailed information about the uses of acetylene, visit the following link.
Table of Contents
1. Physical Properties of Acetylene
Acetylene has a carbon-carbon triple bond (C≡C), characterized by a short length (0.1205 nm) and high energy of formation. This configuration makes acetylene highly unstable and reactive due to its unsaturated nature.
The acidic hydrogen of the C-H bonds (pKa=25) contribute to acetylene’s reactivity. It is more acidic than ethylene (pKa=44) and can form acetylides with strong bases.
Under standard conditions, acetylene is a colorless, nontoxic gas with narcotic properties. Slightly lighter than air, it possesses distinct physical properties outlined in Table 1.
| Property | Value |
|---|---|
| Molecular mass | 26.0379 |
| Critical temperature | 308.32 K (35.17 °C) |
| Critical pressure | 6.139 MPa |
| Critical volume | 0.113 m3/kmol |
| Triple point | 192.4 K (−80.75 °C) |
| Triple point pressure | 128.3 kPa |
| Normal sublimation point and normal boiling point | 189.15 K (−84.0 °C) |
| Crystal transition point | 133.0 K (−140.15 °C) |
| Enthalpy of transition | 2.54 kJ/mol |
| Density |
760.2 kg/m3 (131 K) 764.3 kg/m3 (141 K) |
| Density (liquid C2H2) | 465.2 kg/m3 (273.15 K) |
| Density (gaseous C2H2 at 1 bar) | 1.095 kg/m3 (288.15 K) |
| Molecular volume (0 °C, 1.013 bar) | 22.223 m3/kmol |
| Enthalpy of vaporization (calculated) | 10.65 kJ/mol (273.15 K) |
| Enthalpy of sublimation | 21.168 kJ/mol (5.55 K) |
| Enthalpy of formation | 227.5 ± 1.0 kJ/mol (298.15 K) |
| Gibbs free energy of formation | 209.2 ± 1.0 kJ/mol (298.15 K) |
| Entropy of formation | 200.8 J mol−1 K−1 (298.15 K) |
| Enthalpy of combustion | −1255.6 kJ/mol (298.15 K) |
| Higher heating value | 50 400 kJ/kg |
| Lower heating value | 48 700 kJ/kg |
| Vapor pressure | 2.6633 MPa (273.15 K) |
| Thermal conductivity (0 °C, 1.013 bar) | 0.0184 W m−1 K−1 |
| Heat capacity (ideal gas state) | 43.990 J mol−1 K−1 (298.15 K) |
2. Chemical Reactions of Acetylene
Acetylene is highly reactive towards various elements and compounds because of its high unsaturation and positive free energy of formation. This characteristic makes it a versatile raw material for numerous substances. Key reaction types include addition reactions, hydrogen substitutions, polymerization, and cyclization.
Acetylene is more susceptible to nucleophilic attack compared to ethylene. Additionally, its polarized C-H bond (pKa ≈ 25) grants it acidic properties, facilitating solubility in basic solvents and formation of hydrogen bonds. This behavior deviates from Raoult’s law for solutions containing acetylene.
The development of acetylene pressure reactions by W. Reppe marked a breakthrough in acetylene chemistry. Notable reaction groups include vinylation, ethynylation, carbonylation, and cyclic and linear polymerization.
2.1. Industrially Important Reactions
1. Vinylation:
Vinylation involves the addition of compounds with mobile hydrogen atoms (water, alcohols, thiols, amines, etc.) to acetylene, yielding vinyl derivatives primarily used for polymerization.
Two types exist: heterovinalation (hydrogen from O, S, or N) and C-vinylation (directly bound carbon hydrogen). Examples include acetylene dimerization, trimerization, acrylonitrile synthesis, and addition to reactive unsaturated hydrocarbons like cyclopentadiene.
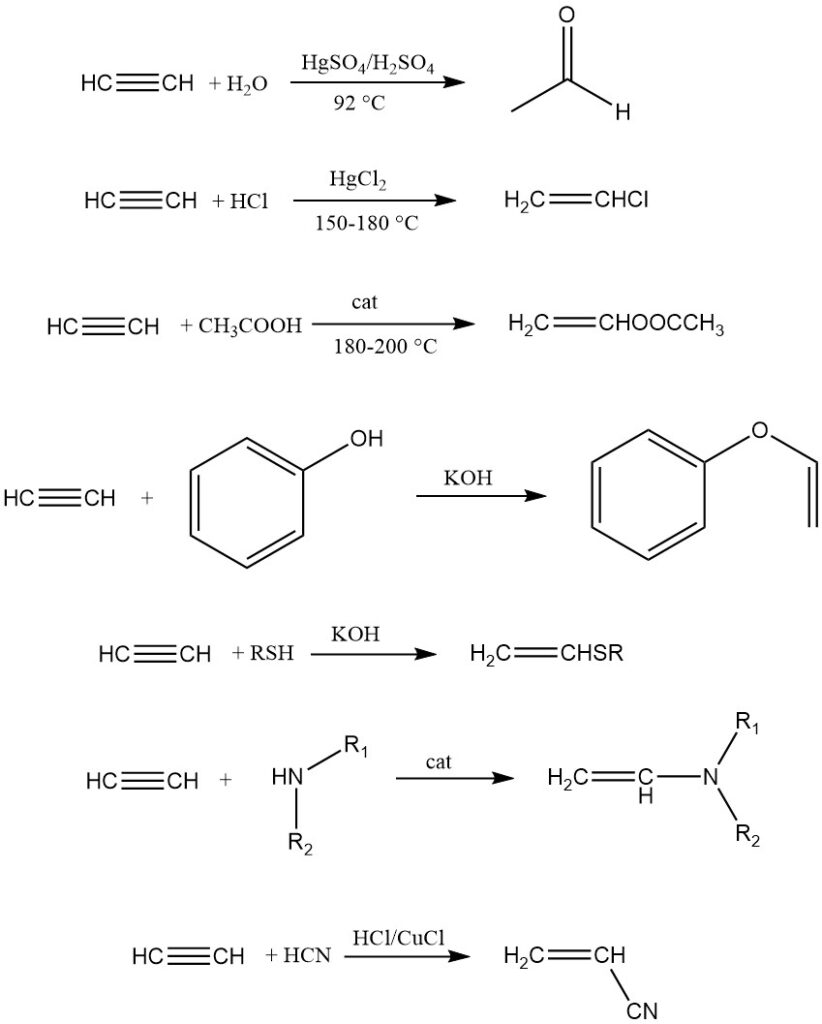
2. Ethynylation:
Ethynylation adds carbonyl compounds to acetylene while preserving the triple bond. Reppe discovered that heavy metal acetylides, particularly Cu2C2·2 H2O·2 C2H2, effectively catalyze the reaction with aldehydes.
Ethynylation of ketones is catalyzed with alkaline catalysts. The general reaction scheme is:

Propargyl alcohol and butynediol are key products of this reaction.

3. Carbonylation:
Carbonylation involves the reaction of acetylene and carbon monoxide with compounds possessing mobile hydrogen atoms, catalyzed by metal carbonyls (e.g., Ni(CO)4). Metal halides capable of forming carbonyls can also be used.
Acrylic acid is a prominent product obtained from acetylene, water, and carbon monoxide using Ni(CO)4 catalyst. Other products of acetylene carbonylation include thioesters of acrylic acid, acrylic amides, carboxylic acid anhydrides and hydroquinone.

4. Cyclization and Polymerization:
Acetylene, under suitable catalysts, can undergo cyclization and linear polymerization. Berthelot first observed cyclization, yielding a mixture of aromatic compounds like benzene and naphthalene. Reppe later synthesized 1,3,5,7-cyclooctatetraene with high yield (70%).
Linear polymerization occurs with copper (I) salts, resulting in vinylacetylene, divinylacetylene, etc. A notable example is cuprene, formed by heating acetylene with copper sponge. Ziegler-Natta catalysts facilitate polyacetylene formation, which exhibits unique properties like conductivity upon doping.

2.2. Other Reactions and Derivatives
1. Metal Acetylides:
Acetylene forms metal acetylides by replacing its hydrogen atoms with metal atoms (M). Alkali and alkaline-earth acetylides are prepared via metal amides or direct reaction with molten or finely divided metals.

Explosive copper acetylides (Cu2C2·H2O) are formed by reaction of copper(I) salts with acetylene in liquid ammonia or copper(II) salts with acetylene in basic solution with reducing agents like hydroxylamine.
Additionally, copper oxides and other copper salts can readily convert to acetylides. Due to this reactivity, copper plumbing should be strictly avoided in acetylene systems. Other explosive metal acetylides exist for silver, gold, and mercury.
2. Halogenation:
Chlorine adds to acetylene with FeCl3 as catalyst to yield 1,1,2,2-tetrachloroethane, an important intermediate in solvent production. Bromine and iodine can also participate in addition reactions, with iodine stopping at 1,2-diiodoethylene.
3. Hydrogenation:
Acetylene undergoes partial or complete hydrogenation over Pt, Pd, or Ni catalysts, resulting in ethylene or ethane.
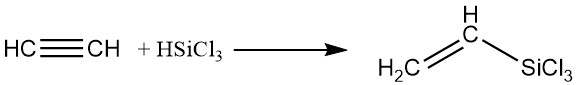
4. Organic Silicon Compounds:
Silanes can be added to acetylene using platinum catalysts in the liquid phase, yielding silicon-containing derivatives.
5. Oxidation:
Acetylene is resistant to oxidation at room temperature but forms explosive mixtures with air or oxygen. Oxidizing agents like ozone or chromic acid convert it to formic acid, carbon dioxide, and other products. Reaction with dilute ozone yields glyoxal.
6. Hydrates:
Under pressure and temperatures below approximately 15 °C, acetylene forms waxy hydrates with the composition C2H2·6 H2O. These clathrate-like structures incorporate water molecules into their crystal lattice, impacting acetylene’s storage and handling. Hydrate formation can cause equipment blockages and pose decomposition risks due to shock waves.
7. Chloroacetylenes:
7.1. Monochloroacetylene (HC≡CCl):
Monochloroacetylene is a gas with a nauseating odor that irritates mucous membranes. It is btained by reacting 1,2-dichloroethylene with alcoholic NaOH in the presence of Hg(CN)2. It is highly flammable and explodes readily in the presence of even trace amounts of oxygen or air and extremely poisonous.
7.2. Dichloroacetylene (ClC≡CCl):
Dichloroacetylene ia a colorless oil with an unpleasant odor that explodes in air or upon heating. It is prepared from acetylene in a strongly alkaline potassium hypochlorite solution or by reacting trichloroethylene vapor with caustic alkali. It is highly explosive and readily decomposes, presenting significant safety concerns.
3. Toxicology of Acetylene
Pure acetylene acts as a simple asphyxiant by displacing oxygen in the atmosphere. However, commercial acetylene no longer contains significant quantities of toxic impurities like arsine, hydrogen sulfide, and phosphine, which historically contributed to adverse health effects from exposure to acetylene produced from carbide calcium.
The lowest documented lethal concentration (LC) for rats is 9% by volume in air. Dogs exhibit greater tolerance, requiring 80% acetylene to induce narcosis with increased blood pressure and decreased pulse rate (indicating vasomotor and vagus nerve stimulation).
In humans, inhaling 10% acetylene produces mild intoxication, while 20% causes significant intoxication and 30% leads to incoordination. Unconsciousness occurs within 5 minutes at 35% exposure.
Lethal inhalation involves 35% for 5-10 minutes or 10% for 30-60 minutes. Symptoms of intoxication include excitement, coma, cyanosis, weak/irregular pulse, and memory loss.
No evidence suggests detrimental health effects from repeated exposure to tolerable acetylene levels. Interestingly, human studies revealed decreased susceptibility upon repeated exposure.
While 33% acetylene induced unconsciousness within 6 minutes initially, subsequent exposures within the same week required longer durations (9 minutes on the second and over 33 minutes on the third) to achieve the same effect.
Unlike some other gases, acetylene does not irritate mucous membranes. Notably, no threshold limit value (TLV) or maximum allowable concentration (MAK) has been established for acetylene.
The current Occupational Safety and Health Administration (OSHA) and National Institute for Occupational Safety and Health (NIOSH) permissible exposure limit (PEL) is 2500 ppm (parts per million) in air.
Reference
- Acetylene; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_097.pub4


