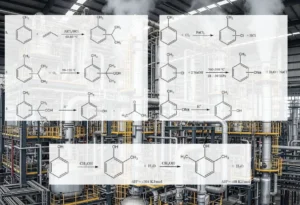
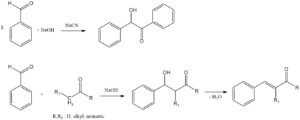
Aromatic amines are produced by three types of reactions:
Reductions: using metallic elements like Iron (Fe), Zinc (Zn), Tin (Sn), Aluminum (Al), or their corresponding salts; sulfur-containing compounds; electrochemical procedures; and catalytic hydrogenation.
Nucleophilic substitutions: involving the exchange of substituents like halogen, hydroxyl, alkoxy, and sulfonic groups.
Rearrangements and degradations: including transformations such as the benzidine and Beckmann rearrangements, along with the Schmidt and Hofmann degradations.
It should be noted that the first two reaction types are more important. Chemical rearrangements and degradations rarely result in pure reaction products with high yields.