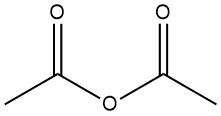
Acetic anhydride is an organic compound with the chemical formula (CH3CO)2O. It is a colorless liquid with a pungent odor and is commonly used as an acetylating agent and a dehydrating agent. It was prepared in 1852 by the reaction of benzoyl chloride and molten potassium acetate.
Since then, acetic anhydride has emerged as a prominent organic intermediate with substantial significance in various research and industrial applications. Currently, it is widely used in both academic and industrial synthesis.
Acetic anhydride is found in trace amounts in wine grapes (Vitis vinifera), but it is mainly produced industrially.
Table of Contents
1. Physical Properties of Acetic anhydride
Acetic anhydride is a colorless liquid characterized by a pungent odor and possesses strong lachrymatory properties. The key physical data associated with acetic anhydride are provided below.
| Property | Value |
|---|---|
| Molecular Formula | (CH3CO)2O |
| Molecular Weight | 102.09 g/mol |
| Appearance | Colorless liquid |
| Density | 1.08 g/cm3 |
| Boiling Point | 140.1 °C |
| Melting Point | -73.1 °C |
| Solubility in Water | Reacts violently; miscible |
| Odor | Pungent |
| Vapor Pressure | 13 mmHg at 25 °C |
| Flash Point | 49 °C (closed cup) |
| Autoignition Temperature | 485 °C |
| Refractive Index | 1.392 |
| Viscosity | 1.3 cP at 25 °C |
| Explosive Limits | 2.3 - 10.6% (volume) |
At a temperature of -73.1 °C, acetic anhydride undergoes a solid-to-liquid phase transition (melting point). On the other hand, it boils at 140 °C under a pressure of 101.3 kPa (boiling point).
Acetic anhydride demonstrates miscibility with polar solvents and exhibits solubility in cold alcohol, albeit with a slow decomposition rate. When dissolved in water at a temperature of 20 °C, the solubility of acetic anhydride is approximately 2.6 wt %, accompanied by a gradual decomposition process.
Conversely, at a temperature of 15 °C, the solubility of water in acetic anhydride is approximately 10.7 wt %, also with a gradual decomposition tendency.
2. Chemical Reactions of Acetic anhydride
Acetic anhydride exhibits a wide range of chemical reactions and has been extensively studied as an aliphatic carboxylic acid anhydride.
In the past 25 years, acetic anhydride has been utilized in numerous publications and patents, particularly for the acetylation of hydroxyl (OH) or amino (NH) groups, serving as a primary step in these reactions.
2.1. Acetylation
Acetic anhydride in pyridine is used to introduce the acetyl group to different moolecules including hydroxy, amino, and thiol groups.
2.1.1. O-Acetylation
Acetic anhydride is highly suitable for esterification reactions with alcohols, which are often challenging or impossible to achieve using acetic acid. During this process, acetic acid is liberated. Catalysts such as bases, strong acids, and salts like sodium acetate are commonly employed.
Notable examples of acetic anhydride’s reaction with hydroxyl groups include the formation of acetyl cellulose, acetylsalicylic acid (commonly known as Aspirin), and glycerol triacetate.
When acetic anhydride reacts with hydrogen peroxide, it produces peracetic acid or diacetyl peroxide, the outcome depending on the molar ratio of the reactants:
(CH3CO)2O + 2 H2O2 → 2 CH3CO3H
(CH3CO)2O + H2O2 → CH3COOH + CH3CO3H
(CH3CO)2O + CH3CO3H → CH3COOOOCOCH3 + CH3COOH
2.1.2. N-Acetylation
The acetylation of compounds containing NH groups yields acetamides, following the general equation:
RR’NH + (CH3CO)2O → RR’NCOCH3 + CH3COOH
Here, R and R’ represent hydrogen (H) or alkyl groups. Aliphatic amines typically react without the need for heating. Aniline, for instance, produces acetanilide, which prevents oxidation during subsequent nitration.
N-Acetylation reactions generally occur faster than acetylation of OH groups, enabling partial acetylation of compounds with multiple functional groups. Examples include the production of N-acetylamino acids like N-acetylmethionine-S-oxide (1) and N-acetyl anthranilic acids (2).
Pyrogallol, 1,2,3-trihydroxybenzene

Amides and carbamides with free NH groups, both aliphatic and aromatic, can be acetylated using acetic anhydride.
Sulfuric acid is commonly used as a catalyst. This reaction finds application in synthesizing various products such as N,N,N’,N’-tetraacetylethylenediamine ((CH3CO)2NCH2CH2N(COCH3)2) and 2,4,6,8-tetraacetylazabicyclo[3.3.1]nonane-3,7-dione.
![2,4,6,8-tetraacetylazabicyclo[3.3.1]nonane-3,7-dione.](https://chemcess.com/wp-content/uploads/2023/07/2468-tetraacetylazabicyclo3.3.1nonane-37-dione.jpg)
2.1.3. C-Acetylation
Compounds with reactive CH bonds can undergo acetylation with acetic anhydride, sometimes requiring a catalyst. Notable examples include the production of ethyl α-cyanoacetoacetate (CH3COCH(CN)COOC2H5) using potassium carbonate as a catalyst and the Friedel-Crafts reaction between acetic anhydride and aromatic hydrocarbons like benzene, resulting in the formation of acetophenone.
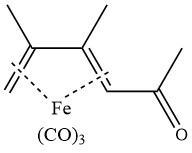
Acetylation of 2,3-dimethylbutadiene tricarbonyl iron in the presence of aluminum chloride yields the following complex:
The reaction of ketones with acetic anhydride, catalyzed by boron trifluoride, leads to the formation of β-diketones.
Triacetylmethane can be directly synthesized from isopropenyl acetate, acetic anhydride, and an aluminum chloride catalyst.
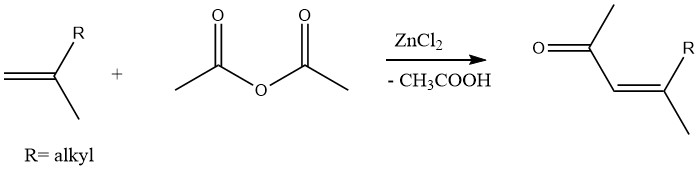
Similarly, unsaturated methyl ketones can be produced through the acetylation of olefins with acetic anhydride in the presence of zinc chloride as a catalyst:
2.1.4. Acetylation of Mineral Acids
The reaction of acetic anhydride with nitric acid results in the formation of acetyl nitrate, frequently employed as a nitrating agent in organic chemistry. Acetyl nitrate can also be synthesized using dinitrogen pentoxide and acetic anhydride.
Other strong acids like sulfuric acid, sulfonic acids, and hydrochloric acid can form mixed anhydrides with acetic anhydride. Reacting acetic anhydride with phosphorous acid produces 1-hydroxyethane-1,1-diphosphonic acid.

2.1.5. Acetylation of Oxides
Antimony trioxide reacts with acetic anhydride to yield antimony triacetate (Sb(OCOCH3)3). A similar reaction with chromium trioxide leads to chromyl acetate solutions, often utilized in the oxidation of olefins and hydrocarbons, but caution must be exercised as they can occasionally be explosive.
2.1.6. Acetylation of Salts
Various salts react with carboxylic acid anhydrides in a manner similar to their corresponding free acids:
Li−C−CR + (CH3CO)2O → CH3CO−C−CR + CH3COOLi
Barium peroxide treated with acetic anhydride produces diacetyl peroxide:
BaO2 + (CH3CO)2O → (CH3CO)2O2 + BaO
A general method for producing vinyl ketones involves the reaction of vinyl magnesium bromides with acetic anhydride.
2.1.7. Production of Acetoxy Silanes
Acetic anhydride reacts with silanes according to the equation:

where X = H, Cl, OR, NR2.
2.1.8. Addition to Heterocyclic Compounds with Ring Cleavage
These reactions are conceptually similar to those described above. For instance, ethylene glycol diacetate can be produced from ethylene oxide in the presence of strongly acidic or basic catalysts, and oxymethylene diacetates can be obtained from trioxane.
2.1.9. Oxidative Addition to Carbon-Carbon Double Bonds
Oxidative addition reactions result in the formation of corresponding diacetates. For instance, the addition of ethylene to acetic anhydride yields ethylene glycol diacetate. Similarly, butadiene can be converted to 1,4-diacetoxy-2-butene through a similar process.
2.1.10. Production of Mixed Diacyl Peroxides
Diacyl peroxides can be synthesized by reacting oxygen with mixtures of aliphatic aldehydes and acetic anhydride in the presence of sodium acetate.
2.1.11. Reaction with N-Oxides
Reactions with N-oxides yield various products based on the specific type of N-oxide. Pyridine-N-oxide, for example, produces 2-acetyloxypyridine, while 4-picoline-N-oxide forms a mixture of 4-acetyloxymethylenepyridine and 3-acetyloxy-4-methylpyridine. The Polonovski reaction of N-oxides results in the formation of formaldehyde, unsaturated aldehydes, and acid amides.
2.1.12. Reaction with S-Oxides
The reduction of sulfoxides to sulfides using acetic anhydride is known as the Pummerer reaction. For example, CH3SOR reacts with acetic anhydride to yield CH3COOCH2SR and CH3COOH.
This reaction is also utilized to oxidize primary and secondary alcohols, even those that are sterically hindered, using mixtures of dimethylsulfoxide or tetramethylene sulfoxide along with acetic anhydride, resulting in the formation of the corresponding carbonyl compounds.
2.1.13. Production of Acylals and Vinyl Acetates
Aldehydes react with acetic anhydride in the presence of acid catalysts to form acylals. If the aldehyde possesses an α-hydrogen atom, elimination of acetic acid leads to the formation of the corresponding vinyl acetate. The addition of zinc facilitates the cleavage of the acetic acid moiety. This process is particularly suitable for the synthesis of 2,2-dichlorovinyl acetate.
2.1.14. Boron Trifluoride
Acetic anhydride and substituted acetic anhydrides can be transformed into acetyl ketones using a boron trifluoride complex that decomposes in warm water.
2.2. Dehydration
Acetic anhydride finds application as a dehydrating agent in various industries, including the explosives industry. One notable example of its dehydrating property is in the production of hexogen (1,3,5-trinitrohexahydro-1,3,5-triazine).

In the synthesis of the nitroester of 1,2,4,5-tetrahydroxy-3,6-dinitrocyclohexane, acetic anhydride is employed for its water-binding capability:

Furthermore, acetic anhydride is utilized in the production of alkyl cyanides by dehydrating aldoximes:
RCH=NOH + (CH3CO)2O → RCN + 2CH3COOH
Additionally, acetic anhydride serves as a dehydrating agent in numerous cyclization reactions.
2.3. Reactions of the α-Protons
The Perkin reaction is a widely employed method for the synthesis of α, β-unsaturated acids from aromatic aldehydes, including benzaldehyde. This reaction typically takes place in the presence of potassium acetate or sodium acetate. It is also used for the production of cinnamalacetic acid.
Similar reactions include oxidative carboxymethylation, where acetic anhydride and oxidizing agents are used, and the conversion of long-chain alkenes into their corresponding carboxylic acid derivatives.
2.4. Reactions of a Single Carbonyl Group
In certain cases, it is feasible for only one of the carbonyl groups in acetic anhydride to participate in a reaction. For instance, when acetic anhydride reacts with hydrogen cyanide in the presence of a base, it forms compound (3). Similarly, in the presence of a Grignard reagent (RMgBr), acetic anhydride yields compound (4).

2.5. Production of Silver Ketenide
When silver acetate and acetic anhydride are reacted in the presence of pyridine at room temperature, they undergo a reaction to form a pyridine complex of silver ketenide. If an excess of acetic anhydride is employed, the resulting mixture can be subjected to fractional distillation of pyridine and acetic acid, leading to the isolation of silver ketenide.
3. Production of Acetic anhydride
Historically, the oldest method of producing acetic anhydride was the conversion of sodium acetate with an excess of an inorganic chloride, such as thionyl chloride, sulfuryl chloride, or phosphoryl chloride.
In this process, half of the sodium acetate is converted to acetyl chloride, which then reacts with the remaining sodium acetate to form acetic anhydride.
Another development was the conversion of acetic acid with phosgene in the presence of aluminum chloride, allowing for continuous operation:
2CH3COOH + COCl2 → (CH3CO)2O + 2HCl + CO2
Two other methods utilized in the past included the cleavage of ethylidene diacetate to form acetaldehyde and acetic anhydride in the presence of acid catalysts like zinc chloride, as well as the reaction of vinyl acetate with acetic acid on palladium(II) catalysts to form acetaldehyde and acetic anhydride. However, these processes are no longer of industrial significance.
Currently, the production of acetic anhydride is primarily carried out through either the ketene process or the oxidation of acetaldehyde. Another method, known as the carbonylation of methyl acetate (Halcon process), was introduced in 1983.
In Western Europe, approximately 77% of acetic anhydride is produced through the ketene process, while 23% is produced through the oxidation of acetaldehyde.
In the United States, since the introduction of the Halcon process at the Tennessee-Eastman plant, 25% of acetic anhydride is produced through this method, while 75% is produced through the ketene process.
3.1. Production of Acetic anhydride by Ketene Process
The ketene process involves two steps: the thermal cleavage of acetic acid to form ketene and the subsequent reaction of ketene with acetic acid to produce acetic anhydride. The overall reactions can be summarized as follows:
1. Thermal cleavage of acetic acid:
CH3COOH → CH2=C=O + H2O (ΔH = 147 kJ/mol)
2. Reaction of ketene with acetic acid:
CH2=C=O + CH3COOH → (CH3CO)2O (ΔH = -63 kJ/mol)
The thermal cleavage of acetic acid to produce ketene and water is carried out by heating hot acetic acid vapor at a temperature of 700-750°C in the presence of traces of phosphoric acid catalyst.
The reactor pressure is typically reduced to allow for the isolation of ketene before it reacts with acetic acid or water. The cleavage process takes place in a multicoil reactor with coils made of highly heat-resistant steel alloys, such as Sicromal.
The ketene process involves multiple steps and equipment configurations, depending on the desired production capacity.
In small-scale operations, separate preheating and cleaving ovens are utilized, while medium-sized ovens are often constructed as single-chamber ovens heated with gas or oil.
Large-scale ovens work effectively with three- or four-chamber systems and partial gas flows. Multiple ovens can also be operated with acetic acid supplied from a central evaporator.
After the thermal cleavage of acetic acid, the resulting ketene is then reacted with acetic acid to produce acetic anhydride. Two processes are commonly used for this reaction: the scrubber process and the Wacker process.
In the scrubber process, ketene is absorbed by circulating glacial acetic acid in scrubbers filled with Raschig rings. The ketene is mostly absorbed in the first scrubber, and the liquid mixture containing raw anhydride is collected and cooled.
In the Wacker process, the ketene is pumped through a liquid-ring pump, where it reacts with acetic acid at controlled temperature and pressure. The raw anhydride is continuously collected, and part of it is returned to the reaction pump.
The raw anhydride obtained from the reaction is further purified through distillation. Both continuous and discontinuous distillation methods are used.
In discontinuous distillation, three fractions are obtained: forerun, middle run, and pure anhydride.
Continuous distillation involves multiple columns to separate the different fractions. The purity of the anhydride is typically around 99% in discontinuous distillation but can be improved by operating under reduced pressure.
The Wacker process is also utilized for the workup process, allowing the processing of waste acids without the need for additional materials. The process has high reliability and energy-saving features.
For the production of 100 kg of acetic anhydride, approximately 122 kg of acetic acid is required, considering the reconcentrated dilute acetic acid. The yield of the process is over 96% at about 75% cleavage.
3.2. Production of Acetic anhydride by Oxidation of Acetaldehyde
Acetic anhydride can also be directly produced by the liquid-phase oxidation of acetaldehyde. This process involves the reaction of peracetic acid, formed from oxygen and acetaldehyde, with a second molecule of acetaldehyde to produce acetic anhydride and water.
Efficient cooling and the use of suitable catalysts are crucial in this process. Metal salt combinations, such as manganese acetate and copper acetate, cobalt acetate and nickel acetate, or cobalt and copper salts of higher fatty acids, are commonly used catalysts.
To maintain the reaction temperature and prevent rapid hydrolysis of acetic anhydride, the process is typically operated between 40°C and 60°C.
Effective cooling is necessary due to the exothermic nature of the reaction, and the addition of low-boiling solvents like methyl acetate and ethyl acetate, which form azeotropic mixtures with water, aids in the separation of water from the reaction mixture.
The ratio of acetic anhydride to acetic acid in the final product depends on the initial ratio of ethyl acetate to acetaldehyde.
In practice, a mixture of acetaldehyde and ethyl acetate is oxidized with the addition of cobalt acetate and copper acetate catalysts. The optimized reaction conditions can lead to a higher acetic anhydride to acetic acid ratio. Various solvents, including methylene chloride, diisopropyl ether, cyclohexanone, and ethylidene diacetate, can be used as diluents in the process.
The gas mixture containing oxygen and acetaldehyde is introduced into the reactor, where the oxidation takes place in the liquid phase in the presence of catalysts. The reactor effluent is then passed through a water-cooled condenser, which also functions as a separator for non-condensable gases.
Fresh acetaldehyde is introduced into a packed column, where it combines with the off-gas from the condenser. The condensates from the condenser and column are distilled to obtain the desired product.
Acetaldehyde is recovered from the non-condensable gas stream. The off-gas may contain combustible low-boiling products and solvents, which can be flared off.
The process described above provides an example, and variations of reactor configurations and specific reaction conditions may be employed by different manufacturers.
3.3. Production of Acetic anhydride by Carbonylation of Methyl Acetate
The conventional process for the production of acetic anhydride involves the thermal decomposition of acetic acid to form ketene, which requires a significant amount of energy.
However, alternative processes have been developed, such as the acetic acid production process by Monsanto and the carbonylation of methyl acetate.
The carbonylation of methyl acetate to form acetic anhydride was patented by Halcon in 1973, and the first plant using this process was operational in 1983. In this process, methyl acetate is carbonylated in the liquid phase at temperatures ranging from 160°C to 190°C and a carbon monoxide partial pressure of 2-5 MPa.
Various catalysts can be used for the carbonylation reaction, including rhodium and nickel compounds activated by iodides or other iodine-containing substances. Rhodium catalysts exhibit higher activity than nickel catalysts, and both catalysts show selectivity above 95%.
The addition of a small percentage of hydrogen to the carbon monoxide used in the synthesis enhances the activity and lifetime of the catalysts. Chromium compounds can also be employed to shorten the induction phase of the reaction.
The carbonylation process can start from either methyl acetate or dimethyl ether, with the latter being converted to methyl acetate before its transformation to acetic anhydride.
The Halcon process (Figure 1), as an example, involves continuously feeding dried methyl acetate into a reactor lined with Hastelloy, where it is carbonylated at 175°C in the presence of a catalyst mixture.

a) Compressor; b) Carbonylation reactor; c) Evaporator; d) Adsorber; e) Distillation column; f) Condenser; g) Scrubber
The heat generated during the reaction is removed by heat exchange and utilized for preheating methyl acetate and generating low-pressure steam. Unreacted carbon monoxide is recycled after the removal of condensable gases.
A side stream is washed with pure acetic anhydride to prevent the buildup of inert gases. The liquid reaction product leaving the reactor undergoes flash distillation to separate catalyst-containing liquid for recycling.
The raw anhydride is further purified through distillation in three consecutive columns, with the final column producing acetic anhydride of 99% purity.
To reduce the iodide content in the pure anhydride, a solution of potassium acetate in acetic anhydride can be added during the distillation process.
These alternative processes offer advantages over the conventional thermal decomposition route in terms of energy consumption and feedstock utilization, allowing the production of acetic anhydride from sources other than oil, such as coal.
4. Uses of Acetic anhydride
Acetic anhydride is used as both an acetylating agent and a dehydrating agent. One prominent application is in the acetylation of cellulose on a large scale. However, there are several other areas where acetic anhydride is employed:
1. Production of poly(methylacrylimide) hard foam, where it acts as a binding agent for liberated ammonia during the conversion of amide groups to imide groups.
2. Acetylated plastic auxiliaries, including glycerol triacetate, acetyl tributyl citrate, and acetyl ricinolate.
3. Explosives production, particularly in the manufacturing of hexogen.
4. Production of certain types of brake fluids.
5. Production of auxiliaries for drilling fluids.
6. Detergent industry, specifically in the production of cold-bleaching activators like tetraacetylethylenediamine.
7. Dyeing industry, where acetic anhydride, in combination with nitric acid, is used as a nitrating agent, making use of its solvent and dehydrating properties.
8. Preparation of various organic intermediates, such as chloroacetyl chloride, diacetyl peroxide, higher carboxylic anhydrides, acetates, and the boron trifluoride complex.
9. Production of pharmaceuticals, including acetylsalicylic acid (aspirin), p-acetylaminophenol, acetanilide, acetophenacetin, theophylline, acetylcholine chloride, sulfonamides, hormones, vitamins, and the x-ray contrast agent 2,4,6-triiodo-3,5-diacetylamidobenzoic acid.
10. Food industry applications, primarily in the acetylation of animal and plant fats to achieve desired solubilities, production of acetostearins (edible packing materials), and clarification of plant oils.
11. Flavors and fragrances, where it is used in the production of esters and coumarin.
12. Herbicides like metolachlor (Dual) and alachlor (Lasso).
References
- Acetic Anhydride and Mixed Fatty Acid Anhydrides; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_065
- https://lotus.naturalproducts.net/compound/lotus_id/LTS0216280
Frequently Asked Questions about Acetic Anhydride
Acetic anhydride is an organic compound with the chemical formula (CH3CO)2O. It is a colorless liquid with a pungent odor and is commonly used as an acetylating agent and a dehydrating agent.
Acetic anhydride reacts violently with water, alcohols, and other substances that contain active hydrogen atoms. This reaction can be exothermic and may lead to fire or explosion hazards.
Acetic anhydride has various applications, including:
– Acetylation of cellulose in the production of cellulose acetate, a material used in fibers, films, and plastics.
– Production of pharmaceuticals, such as acetylsalicylic acid (aspirin), acetaminophen, and certain hormones.
– Manufacturing of chemicals, including acetylating agents, higher carboxylic anhydrides, and certain esters.
– Synthesis of flavors, fragrances, and herbicides.
– Use as a dehydrating agent in various industrial processes.
When working with acetic anhydride, it is important to follow these safety precautions:
– Wear appropriate personal protective equipment (PPE), including gloves, goggles, and a lab coat, to protect against skin and eye contact.
– Work in a well-ventilated area or use fume hoods to prevent inhalation of vapors.
– Keep acetic anhydride away from open flames, sparks, or sources of ignition as it is flammable.
– Handle with care to avoid contact with water or other reactive substances.
– Store acetic anhydride in a cool, dry, and well-ventilated area away from incompatible materials.
Acetic anhydride is a better acylating agent than acetic acid because it readily donates an acetyl group (CH3CO) to reactants. Acetic anhydride reacts more efficiently due to the absence of water, which can hinder the acylation reaction. The absence of water in acetic anhydride allows for higher yields and faster reaction rates in acylation reactions.
The density of acetic anhydride is approximately 1.08 grams per cubic centimeter (g/cm³) at room temperature.
When salicylic acid reacts with acetic anhydride in the presence of an acid catalyst, such as sulfuric acid, it undergoes an acetylation reaction. The acetic anhydride donates an acetyl group to the hydroxyl group of salicylic acid, resulting in the formation of acetylsalicylic acid, commonly known as aspirin.
Acetic anhydride itself is not inherently explosive. However, it can form explosive mixtures with air when its concentration reaches certain levels. Acetic anhydride should be handled with caution due to its reactivity and flammability. It is important to store and handle acetic anhydride following proper safety protocols to minimize the risk of accidents.


