1,3-Propanediol is a transparent, colorless, and odorless liquid that is miscible with water, alcohols, ethers, and formamide. It has limited solubility in benzene and chloroform.
This compound serves as an important monomer in the manufacture of polyesters, polyurethanes, and polyethers.
In recent years, the demand for 1,3-propanediol has experienced significant growth, and the discovery of novel production methods has resulted in a decrease in its cost.
Table of Contents
1. Production of 1,3-Propanediol
1.1. Hydrogenation of 3-Hydroxypropanal
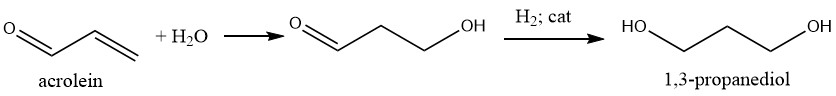
1,3-Propanediol can be synthesized through a two-step process involving the hydrolysis of acrolein to 3-hydroxypropanal followed by hydrogenation.
During hydrolysis, water is employed under weakly acidic conditions, with initial acrolein concentration in water at around 20%. Elevated acrolein concentrations tend to generate more undesirable byproducts due to reaction between acrolein and hydroxypropanal.
Direct hydrogenation of 3-hydroxypropanal in the aqueous phase is possible, although the preferred method involves extracting the aldehyde into an organic solvent, mainly 2-methylpropanol, and subsequently hydrogenating it to yield the diol.
Hydrogenation can be carried out using Raney nickel under pressure in the aqueous phase or nickel-supported catalysts at 2-4 MPa and 110-150°C in the organic phase.
The diol can be separated from the solvent and water through distillation. However, the yield of the desired product is relatively low, at approximately 45%, using this route.
Careful control is necessary for handling acrolein in the production process.
Degussa-DuPont used this process for 1,3-propanediol production until the early 2000s. However, it has been replaced by biotechnological production due to the handling hazards and concerns about sustainability.
1.2. Hydroformylation of Ethylene Oxide
A method that has been thoroughly investigated by Shell to synthesize 1,3-propanediol is the hydroformylation of ethylene oxide.
In the initial step, carbon monoxide and ethylene oxide react in the presence of an organometallic catalyst to generate 3-hydroxypropanal, which is subsequently reduced via hydrogenation to obtain 1,3-propanediol in high yield (92%).
This method provides a slight advantage as ethylene oxide is less expensive than 3-hydroxypropanal. However, a disadvantage of both processes is the production or use of hazardous substances, as well as the requirement for high pressures and temperatures.

1.3. Selective Deoxygenation of Glycerol
Glycerol may be transformed to propanediols by using heterogeneous or homogeneous catalysis.
When employing an organometallic catalyst on a zirconium dioxide support at an elevated pressure of hydrogen and 170°C, selective deoxygenation of glycerol results in a mixture of 1,3-propanediol (24% yield), 1,2-propanediol (12% yield), and 1-propanol (28% yield).
Using p-toluenesulfonic acid as a catalyst and protecting groups, an overall yield of 72% for 1,3-propanediol can also be achieved. This process involves acetalization to protect the first and third hydroxyl groups of the glycerol, tosylation of the central hydroxy group to make it a better leaving group, followed by detosylation and hydrogenolysis.

2. Chemical reactions of 1,3-propanediol
1,3-Propanediol displays the typical properties of alcohols. At elevated temperatures, it can condense with carboxylic acids to form esters, similar to 1,2-propanediol.
Additionally, it can react with acid chlorides and isocyanates to form esters and urethanes, respectively. Unlike 1,2-propanediol, 1,3-propanediol possesses two primary hydroxyl groups that exhibit equivalent reactivity.
1,3-propanediol tends to easily form ethers. Specifically, upon prolonged reflux, 3,3´-Dihydroxydipropyl ether can be produced. 1,3-Propanediol can also form 1,3-dioxanes upon reaction with aldehydes and ketones, often in the presence of acidic catalysts.
When exposed to aluminum oxide at temperatures above 250°C, 1,3-propanediol decomposes into allyl alcohol, propanol, and other byproducts.
It can also be useed to form polyesters with diacids, and the resulting polyester with terephthalic acid is known to exhibit a crystalline melting point of 220°C.
Polyurethanes can also be synthesized from 1,3-propanediol.
Due to its low toxicity and readily biodegradable nature, 1,3-propanediol is expected to have a low environmental impact.
3. Uses of 1,3-propanediol
1,3-Propanediol, being a diol, shares numerous polymeric applications with other low molecular mass diols such as ethylene glycol, 1,2-propanediol, and 1,4-butanediol.
However, its utilization was limited to applications demanding highly specific performance characteristics for a significant period due to its relatively elevated cost. Nonetheless, the development of cost-effective production routes has extended its utility.
One of the most significant applications is its use as a starting material to produce polytrimethylene terephthalate (PTT).
Moreover, 1,3-propanediol is employed as an antifreeze agent and coolant.
It serves as a raw-material source for 1,3-dioxanes.
1,3-Propanediol-bis(4-aminobenzoate) can function as a chain extender in polyurethane elastomers. This bisbenzoate, which can be synthesized from 1,3-dichloropropane as well, finds additional applications as a cross-linking agent in epoxy formulations and as a rubber additive.
Reference
- Propanediols, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a22_163.pub2