1,4-Butanediol (often abbreviated as 1,4-BDO) is a chemical compound with the molecular formula C4H10O2. It is a colorless and odorless liquid that belongs to the family of diols, which are compounds that contain two hydroxyl (OH) groups.
Table of Contents
1. Physical Properties of 1,4-Butanediol
1,4-Butanediol (molar mass: 90.12 g/mol) is a transparent liquid with minimal odor, displaying hygroscopic properties. It readily dissolves in water, alcohols, ketones, glycol ethers, and glycol ether acetates. However, its solubility in diethyl ether and esters is lower, and it is not soluble with aliphatic and aromatic hydrocarbons as well as chlorinated hydrocarbons.
| Property | Value |
|---|---|
| Melting Point | 20.2°C |
| Boiling Point | 230.5 °C (at 101.3 kPa) |
| Density (𝜚) | 1.017 g/cm³ (at 20 °C) 1.0154 g/cm³ (at 25 °C) |
| Critical Temperature (tc) | 446 °C |
| Critical Pressure (pc) | 41.2 bar |
| Viscosity (𝜂) | 91.56 mPa s (at 20 °C) 71.5 mPa s (at 25 °C) |
| Refractive Index (nD) | 1.4460 (at 20 °C) 1.4446 (at 25 °C) |
| Dielectric Constant (𝜀) | 31.4 |
| Flash Point | 134 °C |
2. Chemical Reacction of 1,4-Butanediol
1,4-Butanediol readily undergoes cyclization when exposed to an acidic medium, producing tetrahydrofuran.
When subjected to dehydrogenation in the gas phase and using copper-based catalysts, it forms 𝛾-butyrolactone.
Furthermore, 1,4-Butanediol reacts with monocarboxylic acids, yielding diesters. Esterification with dicarboxylic acids and their derivatives results in the formation of partially crystalline, linear, thermoplastic polymeric esters.
Around 200 °C, 1,4-butanediol reacts with ammonia or an amine using nickel or cobalt catalysts and in the presence of hydrogen, yielding pyrrolidine or pyrrolidine derivatives.
Phosgene reacts with 1,4-butanediol at -5 °C, producing the bis(chloroformate) of butanediol.
Additionally, acrylonitrile adds to 1,4-butanediol at temperatures between 20 and 100 °C, with the presence of catalytic amounts of alkali, leading to 1,4-bis(2-cyanoethoxy)butane.
Similar to other alcohols, 1,4-butanediol can also undergo vinylation, resulting in the formation of mono or divinyl ether.
3. Production of 1,4-Butanediol
Since the 1990s, various alternative technologies have been developed for the production of 1,4-butanediol. The Reppe technology, which relies on acetylene and formaldehyde, remains the most prevalent method.
Additionally, other process utilize benzene or butane via maleic anhydride, propylene, butadiene, and sugars as alternative routes for its manufacturing.
3.1. 1,4-Butanediol from 2-Butyne-1,4-diol
1,4-Butanediol is manufactured on a large industrial scale by continuous hydrogenation of 2-butyne-1,4-diol. This process predominantly employs modified nickel catalysts. The two-stage flow process is carried out within the temperature range of 80-170 °C and at a pressure of 250-300 bar.

The process starts with an aqueous solution of 2-butyne-1,4-diol (30-50%), along with carbon monoxide-free hydrogen and recycled reaction mixture, acting as a heat dissipating medium.
This mixture passes through a reduced nickel-type fixed-bed catalyst in the lead reactor, where more than 99% of the reaction takes place.
The initial temperature is approximately 80 °C, and it should not exceed 170 °C to avoid unwanted decomposition reactions. Hydrogen circulation ensures better liquid distribution. The second or finishing reactor completes the conversion of unsaturated compounds.
Different commercially employed processes primarily differ in the catalyst used. BASF and Invista Technologies describe Ni supported on oxides of zirconium or aluminum, while Raney nickel, employed as a fixed-bed catalyst, is used and licensed by Invista.
Another variation involves low-pressure hydrogenation at around 20 bar using a suspended Raney nickel catalyst, followed by hydrogenation at 120-140∘C and 140-210 bar catalyzed by a fixed-bed contact (by Ineos).
The crude product obtained contains methanol, propanol, and butanol as byproducts, along with traces of 2-methyl-1,4-butanediol, 4-hydroxybutyraldehyde, 𝛾-butyrolactone, acetals, and triols.
To obtain pure 1,4-butanediol, the reactor effluent undergoes fractional distillation. Initially, water and monoalcohols like methanol, propanol, and butanol are removed. Subsequently, 1,4-butanediol is separated from high-boilers such as triols and salts, along with impurities like 𝛾-butyrolactone, acetals, 2-methyl-1,4-butanediol, and 4-hydroxybutyraldehyde.
Pure 1,4-butanediol can be further isolated in an additional column, which separates 4-hydroxybutyraldehyde, acetals, and 𝛾-butyrolactone as light boilers.
3.2. 1,4-Butanediol Based on Benzene or Butane Via Maleic Anhydride
The gas-phase oxidation of benzene or n-butane with oxygen leads to the production of maleic anhydride. This maleic anhydride can be adsorbed from the oxidation off-gas by water, resulting in maleic acid, or by organic high-boiling compounds such as dibutylphthalate.
The maleic acid solution in water can be hydrogenated in a high-pressure process to yield 1,4-butanediol, with the presence of doped Re-based active carbon catalysts. The dopants used in these catalysts are mainly Pd and Pt.
However, the process generates byproducts such as n-butanol, n-propanol, methanol, and tetrahydrofuran. Despite its simplicity, the hydrogenation catalyst is expensive due to the high content of precious metals.
Consequently, only one commercial production of this process has been realized so far, conducted by Lima in the USA.
A more common commercial approach involves the gas-phase hydrogenation of maleic diesters, which are derived from maleic anhydride.
This process was developed by Kvaerner Process Technology (KPT, London, now Johnson Matthey Davy Technologies (JM Davy)) in the 1980s. The first step involves producing dimethyl maleate from maleic anhydride and methanol, using a strongly acidic ion exchange resin as a catalyst.
The resulting dimethyl maleate is then hydrogenated in the gas phase using a Cu-containing catalyst system at a pressure of 2-8 MPa and temperatures of 150-250 °C.
This process yields a mixture of 1,4-butanediol, tetrahydrofuran, butyrolactone, a small amount of the intermediate dimethyl succinate, and, as the major byproduct, n-butanol.
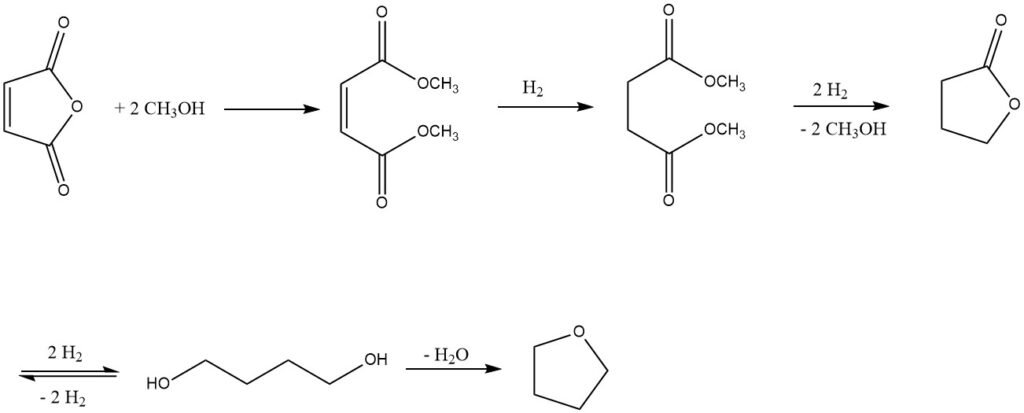
Butyrolactone and dimethyl succinate can be recovered as an azeotrope and recycled back to the hydrogenation stage to achieve complete conversion to 1,4-butanediol and tetrahydrofuran.
JM Davy has licensed this process multiple times to establish butanediol/butyrolactone/tetrahydrofuran plants in various locations in Asia, including Saudi Arabia, Malaysia, Korea, and China.
3.3. 1,4-Butanediol from Propylene
An alternative feedstock for 1,4-butanediol is propylene, which can be obtained via allyl alcohol. Dairen in Taiwan and China, as well as Lyondell in the USA and Netherlands, utilize this process.
To obtain allyl alcohol, propylene is first oxidized to propylene oxide, which is then isomerized (Lyondell) or hydrolyzed to allyl alcohol. The hydrolysis involves acetoxylation of propylene in the gas phase with oxygen, leading to the formation of allyl acetate, which is subsequently hydrolyzed to yield allyl alcohol (Dairen).
The next step involves hydroformulation of allyl alcohol in a solvent with a homogeneous rhodium-based catalyst, producing 4-hydroxybutyraldehyde. A major byproduct in this step is 3-hydroxy-2-methyl-propionaldehyde.
After extracting the aldehydes, they are subjected to hydrogenation using Raney nickel as the catalyst, resulting in 1,4-butanediol, which is then purified by distillation.
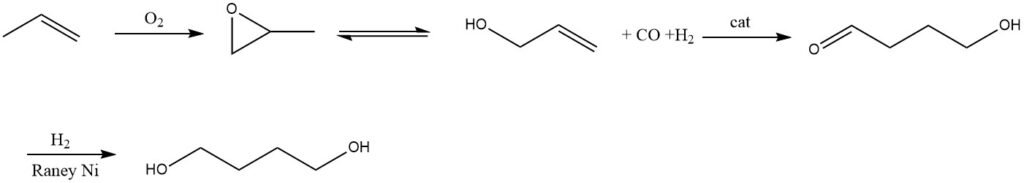
However, one of the major byproducts in this reaction is 2-methyl-1,3-propanediol. Additionally, other byproducts formed during this process include n-propanol and isobutyraldehyde.
3.4. 1,4-Butanediol from Butadiene
Mitsubishi employs a three-step process to produce 1,4-butanediol:
- Butadiene and acetic acid undergo a catalytic reaction to yield 1,4-diacetoxy-2-butene.
- Subsequent hydrogenation of 1,4-diacetoxy-2-butene gives 1,4-diacetoxybutane.
- Finally, hydrolysis of 1,4-diacetoxybutane leads to the formation of 1,4-butanediol.
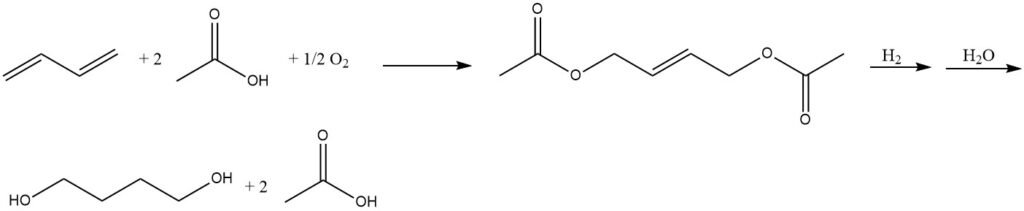
The hydrolysis of the diacetate can also yield tetrahydrofuran due to the highly acidic reaction conditions.
BASF has patented a similar process, where acetic acid first adds to butadiene, and the resulting product isomerizes to 1,4-diacetoxy-2-butene.
Toyo Soda’s process involves the addition of chlorine to butadiene to form a mixture of 1,4-dichloro-2-butene and 3,4-dichloro-1-butene. This mixture reacts with sodium acetate to produce 1,4-diacetoxy-2-butene, which is then directly hydrogenated to 1,4-butanediol.
In a patent described by Shell, butadiene reacts with tert-butyl hydroperoxide using a cobalt catalyst to form 1,4-di(tert-butylperoxy)butene-2, which is then converted to 1,4-butanediol by hydrogenation. As a byproduct, 1,2-butanediol is formed.
Eastman has a different process that starts with butadiene as the raw material. First, butadiene is oxidized by oxygen in the presence of a silver catalyst to form 1,2-epoxy-3-butene. The epoxide then undergoes rearrangement to yield 2,5-dihydrofuran, which is subsequently isomerized to 2,3-dihydrofuran.
After acid-catalyzed addition of water to an equilibrium of 2-hydroxytetrahydrofuran and 4-hydroxybutyraldehyde, followed by hydrogenation, 1,4-butanediol is generated. Alternatively, the conversion of 2,5-dihydrofuran to 1,4-butanediol can be performed as a one-pot synthesis.
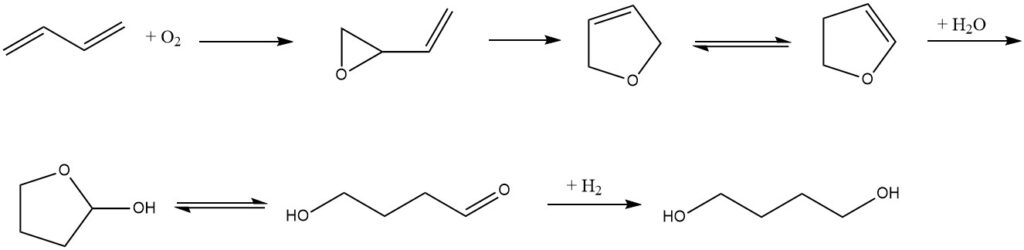
3.5. 1,4-Butanediol from Biomass
In the last two decades, significant efforts have been made to produce 1,4-butanediol and its derivatives from biomass sources.
One of the early approaches involved using furfural, which can be decarbonylated to furan.
BASF developed a promising method where furan is hydrogenated in the presence of water and a rhenium-based catalyst at elevated pressure, resulting in the formation of 1,4-butanediol. 𝛾-Butyrolactone and n-butanol are produced as byproducts.
Shell also worked on optimizing the process using rhenium-based catalysts. With a Re/Pd on carbon catalyst, Shell achieved a high yield of tetrahydrofuran and 1,4-butanediol as major products.
Some companies use fermentation methods based on sugar to directly obtain 1,4-butanediol or its precursors. Genomatica developed a process to produce 1,4-butanediol by fermenting glucose using genetically modified Escherichia coli bacteria. After fermentation, the broth contains 1,4-butanediol diluted in water, along with biomass and salts.
A distillation process separates water, and after thorough filtration and removal of salts, the crude 1,4-butanediol undergoes hydrogenation to eliminate impurities that may affect color stability.
Further purification is carried out by additional distillation steps. The Genomatica process has been commercially implemented in one plant (30 ×103 t capacity, Novamont) in Italy since 2016.
Another approach involves fermenting sugars to succinic acid, which serves as a precursor for 1,4-butanediol production. Companies like BioAmber, Myriant, Reverdia, and Succinity have been involved in commercializing this fermentation process.
The direct fermentation of sugars to succinic acid requires pH control due to the resulting low pH of the fermentation broth. Basic materials like ammonia or alkali/earth alkali hydroxides are used for pH control.
The resulting diammonia succinate can be treated with sulfuric acid to free succinic acid. Succinic acid can then be converted to 1,4-butanediol by hydrogenation in the gas phase as an anhydride or diester using Cu-based catalysts or through liquid-phase hydrogenation using precious metal-based catalysts.
Sugars fermentation can also lead to 𝛾-butyrolactone. Metabolix developed a method to convert sugars in a fermentation process to poly(4-hydroxybutyrate) (poly(butyrolactone)), which can be depolymerized to butyrolactone in the presence of a catalyst at temperatures above 200 °C.
The best results are achieved with calcium hydroxide as a catalyst, and the residual biomass is converted into solid fuel. However, no commercial plant has been established for this process yet.
4. Uses of 1,4-Butanediol
1,4-Butanediol plays a crucial role as a versatile intermediate in the chemical industry and serves as a precursor for 𝛾-butyrolactone and tetrahydrofuran.
Its most significant application lies in the production of polyurethanes and polyesters, such as poly(butylene terephthalate), which is commonly known as PBT. These materials have diverse uses in various industries.
Polyurethanes derived from 1,4-butanediol are employed in the production of cellular and compact elastomers, which find wide-ranging applications due to their elasticity and resilience.
Poly(butylene terephthalate) is extensively processed to create plastic materials and hot-melt adhesives. Additionally, it is utilized in the production of plastic films and fibers, making it a versatile material used in different products and industries.
References
- Butanediols, Butenediol, and Butynediol, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_455.pub2
-
Organic Acids: Succinic and Malic Acids. – https://www.sciencedirect.com/science/article/abs/pii/B9780444640468001592
- https://www.industrialchemicals.gov.au/sites/default/files/1-4-Butanediol.pdf
FAQ
1,4-Butanediol (BDO) is a chemical compound with the molecular formula C4H10O2. It is a colorless, odorless, and hygroscopic liquid with a wide range of applications in various industries.
1,4-Butanediol can be produced by several methods. The most common industrial process is the hydrogenation of 2-butyne-1,4-diol, maleic anhydride or maleic acid. Other methods include the catalytic reaction of butadiene and acetic acid or fermentation of sugars using genetically modified bacteria.
1,4-Butanediol has diverse uses across different industries. Some of its main applications include:
– Production of polyurethanes and polyesters, including poly(butylene terephthalate).
– Solvent in plastics, resins, and coatings manufacturing.
– Cleaning and degreasing agent in industrial and household products.
– Chemical intermediate in the synthesis of γ-butyrolactone (GBL) and tetrahydrofuran (THF).
1,4-Butanediol is also known by its abbreviation “BDO.” It is a common shorthand used in the chemical industry to refer to this compound.


