
Hydrazine is an inorganic compound with the formula N2H4. It is a highly toxic colorless liquid with a strong ammonia-like odor.
Hydrazine is predominantly sold in the form of an aqueous solution containing up to 64% concentration, corresponding to hydrazine hydrate N2H4·H2O.
In 1875 EMIL FISCHER made a prediction regarding the presence of hydrazine H2N–NH2. In 1887, CURTIUS succeeded in isolating it. Then, in 1893, DE BRUYN managed to isolate anhydrous hydrazine.
RASCHIG invented the initial commercial production process in 1907, which is still utilized in Japan, Russia, China, and Korea.
The extensive use of hydrazine and its derivatives as blowing agents for plastic foams led to the emergence of various industrial applications such as boiler water treatment, polymerization initiators, pesticides, pharmaceuticals, photographic chemicals, and dyes.
Even after a century of its discovery, the synthesis of hydrazine remains challenging primarily due to thermodynamic constraints. The majority of hydrazine is manufactured using modified versions of the Raschig process, involving the oxidation of ammonia by hypochlorite.
Nevertheless, newer plants constructed since 1980 employ the PCUK process, which utilizes hydrogen peroxide as the oxidant.
Table of Contents
1. Physical Properties of Hydrazine
Hydrazine is a colorless liquid with an ammonia-like smell. It exhibits complete miscibility with water and forms highly alkaline aqueous solutions.
Notably, specific properties such as viscosity and density reach their maximum values when the composition is at 64%, indicating the presence of the monohydrate form, N2H4·H2O, in both the solid and liquid phases.
Hydrazine forms an azeotrope (boiling point 120.5 °C) with water, where the azeotropic mixture contains 58.5 mol % hydrazine.
Hydrazine is an endothermic compound, with a heat of formation of +50.6 kJ/mol. Its explosive limits in air range from 4.7% to 100%. The upper limit indicates that anhydrous hydrazine is capable of self-explosion.
However, diluting it with an inert gas like nitrogen or water significantly narrows down the flammable range by increasing the lower explosive limit. Consequently, hydrazine hydrate (containing 30.9 vol% hydrazine) can be safely handled at atmospheric pressure and 120 °C in the absence of air.
An overview of certain physical properties of hydrazine is provided below:
- Molar mass = 32.05 g/mol
- fusion point = 2 °C
- boiling point = 113.5 °C
- density = 1.0045 g/ml
- Refractive index = 1.4644 at 25 °C
- viscosity = 0.974 μPa.s
- pH (at 65% water solution) = 12.75
2. Chemical reactions of hydrazine
The chemical properties of hydrazine are heavily influenced by the following characteristics: its endothermic nature, alkalinity, and strong reducing agent properties.
2.1. Thermal Decomposition
Significant decomposition of hydrazine requires a relatively high temperature (250 °C) in the absence of catalysts. However, the presence of certain catalysts such as copper, cobalt, molybdenum, and their oxides lowers the decomposition temperature. Therefore, cautious handling of hydrazine is necessary.
2.2. Acid – Base Reactions
Hydrazine acts as a weak base and reacts with water:

The N2H6+ cation is only observed in highly acidic solutions or in the solid state.
Hydrazine can form salts with acids, including explosive ones like nitrate, perchlorate, and azide. On the other hand, commercially available salts like hydrochloride, hydrobromide, and sulfate can be handled similarly to hydrazine hydrate.
2.3. Reducing Agent
Hydrazine displays strong reducing agent properties and exothermically reacts with oxygen:

Many applications of hydrazine are based on this reaction. The oxidation of hydrazine by air in alkaline solutions is catalyzed by several metals. Therefore, hydrazine solutions should be distilled in the absence or deactivation of copper, polyvalent metals, or their salts. The oxides of cadmium, magnesium, zinc, and aluminum provide stabilization against aerial oxidation in hydrazine solutions.
In acidic solutions, hydrazine reacts with halogens:

These reactions find utility in determining N2H4 (using iodine), purifying crude hydrogen halides, and removing trace amounts of halogens in wastewater. The same procedure can be employed to remove traces of hydrazine itself. Sodium hypochlorite or hydrogen peroxide, in the presence of iron(III) or copper(II) salts, are convenient options for waste or spill treatment.
Hydrazine can reduce various metal ions or oxides such as copper, silver, gold, mercury, nickel, and platinum, transforming them into pulverulent metals.
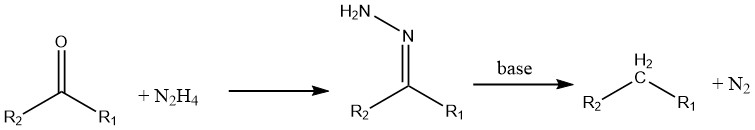
Ketones and aldehydes undergo reduction in the presence of hydrazine (known as the Wolff – Kishner reaction).
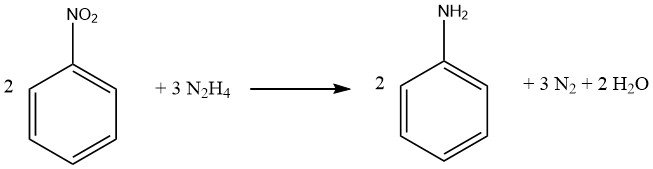
Aromatic nitro compounds can be reduced to their corresponding amines using hydrazine and a hydrogenation catalyst like Raney nickel.
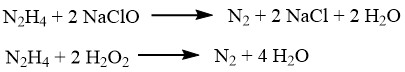
Hydrazine undergoes oxidation to diimide in the presence of hydrogen peroxide. Diimide then reduces acetylenes to cis-alkenes and hydrogenates residual double bonds in acrylonitrile – butadiene rubber.

2.4. Diamine Reactions
Hydrazine finds extensive use in the synthesis and production of various open-chain and heterocyclic nitrogen compounds, including hydrazo and azo compounds, pyrazoles, triazoles, urazoles, tetrazoles, pyridazines, and triazines.
3. Uses of Hydrazine
Most of the hydrazine available is sold as a solution in water. Anhydrous hydrazine, on the other hand, is primarily used as rocket fuel or as a mono- or bipropellant for satellites and spacecraft.
Approximately 80-90% of hydrazine production is converted into organic derivatives. Its other applications are based on its role as a reducing agent, an energy-rich compound, or its hydrogen storage capacity.
The significant uses of hydrazine and its derivatives include their use as polymerization initiators and blowing agents for foamed plastics, as well as in pesticide production. They are also utilized as synthetic building blocks, pharmaceuticals, propellants, and airbags for cars.
3.1. Blowing Agents
Industrial production of hydrazine-based blowing agents is substantial, with hydrazine hydrate consumption for this purpose reaching 50,000 tons per year in 1998. These blowing agents are mainly hydrazo or azo derivatives, with the latter obtained through oxidation of the former using chlorine or hydrogen peroxide.
When heated, blowing agents decompose, releasing nitrogen and other gases, which create a foaming effect in polymers, resulting in the formation of pores or cells. Various commercially produced hydrazine-based blowing agents are available, and their decomposition temperature depends on factors such as particle size, pH, and the presence of activating agents like barium, cadmium, or zinc salts.
One example of a commercial blowing agent is azobis(isobutyronitrile) (AIBN), used for sponge rubber products and PVC foams. It also acts as a free radical source in polymerization initiation. AIBN is synthesized from acetone cyanohydrin and hydrazine, followed by oxidation with chlorine.
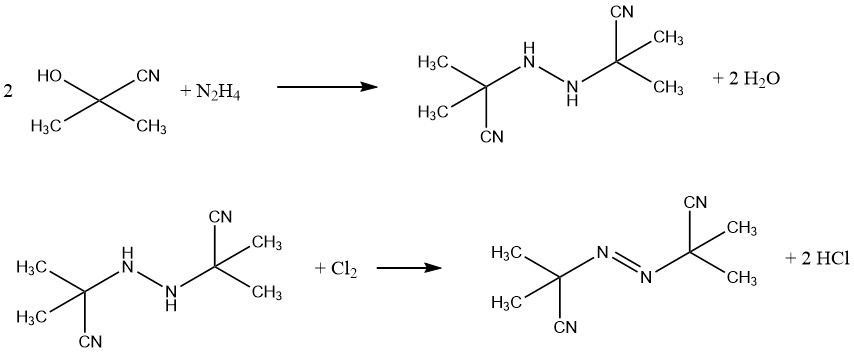
Azodicarbonamide is another significant blowing agent produced from urea and hydrazine.

It intermediate hydrazodicarbonamide can be obtained directly from urea and sodium hypochlorite or via oxidation with chlorine or hydrogen peroxide in the presence of a small amount of bromide ions in an acidic medium.
Azodicarbonamide is the most widely used blowing agent due to its large gas volume evolved upon decomposition and its safe characteristics.
To meet the requirements of manufacturing new rubber or porous plastic materials, sulfonic acid hydrazides were introduced in the 1950s.
These hydrazides are superior to previously used inorganic blowing agents (e.g., ammonium nitrite, ammonium bicarbonate, sodium bicarbonate) in terms of dispersion, higher temperature tolerance, and improved foam cell structure.
They are also colorless, odorless, and safe, with decomposition products that do not support combustion. Sulfonic acid mono- and dihydrazides are produced from hydrazine hydrate and the corresponding sulfonic acid chloride.
3.2. Airbags
Sodium azide is commonly used as a gas precursor in airbag technology. One manufacturing process for sodium azide involves hydrazine and an alkyl nitrite. Another hydrazine derivative, 5-aminotetrazole, obtained from aminoguanidine salts, is being developed for the same application.
3.3. Free-Radical Polymerization Initiators
Azo compounds, particularly symmetrical azodinitriles, are widely used as free radical polymerization initiators.
These azo compounds are synthesized from hydrazine, a ketone, and hydrogen cyanide (HCN). The hydrazo derivative is oxidized using chlorine or hydrogen peroxide in the presence of a bromide catalyst.
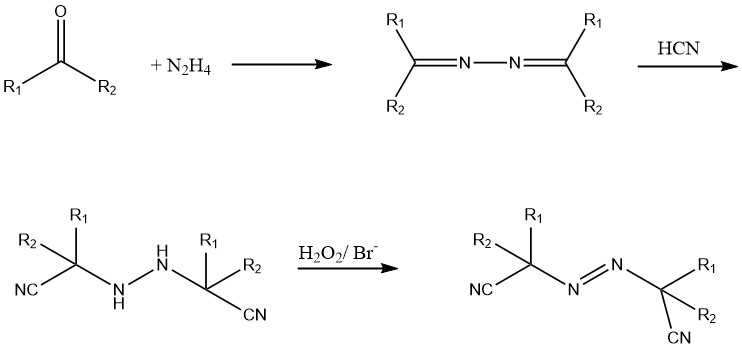
Water-soluble compounds like 2,2′-azo bis(2-aminopropane) dihydrochloride and liquid azo compounds like diethyl 2,2′-azobisisobutyrate are currently under development.
3.4. Pesticides
Hydrazine-based pesticides constitute a significant portion of hydrazine consumption. The first example is maleic hydrazide, synthesized by reacting maleic anhydride with hydrazine.
Another general-purpose herbicide is 3-amino-1,2,4-triazole, obtained from cyanamide, hydrazine hydrate, and formic acid. This herbicide is selectively used in vineyards and orchards, with consumption reaching several thousand tons per year.
Numerous hydrazine-based compounds are commercially produced for pesticide applications, mainly heterocyclic compounds like triazines, oxadiazoles, pyrazoles, pyridazines, and thiadiazoles.
3.5. Pharmaceuticals
Although representing a small percentage of total hydrazine production, the use of hydrazine in pharmaceuticals is significant. For example, isoniazid, the hydrazide of isonicotinic acid, was first employed in the 1950s for treating tuberculosis.
In the 1980s and 1990s, other hydrazine-based pharmaceuticals containing the 1,2,4-triazole group were introduced, serving as antidepressants, antihypertensives, and antibacterial or antifungal agents.
Recent developments have focused on pharmaceuticals containing the 4-amino-1,2,4-triazole group, which demonstrate improved efficiency.
3.6. Water Treatment
Hydrazine is used in water treatment to protect steel from corrosion in boilers. When hydrazine reacts with iron(III) oxide, it forms magnetite, which serves as a protective layer against corrosion by water and oxygen.
Residual hydrazine concentrations below 0.1 ppm ensure full corrosion protection. Commercially available catalyzed hydrazine hydrate formulations, known as activated hydrazine, are effective even at room temperature.
3.7. Propellants
Hydrazine’s initial large-scale use was as a rocket fuel. Anhydrous hydrazine is an excellent propellant, with only hydrogen surpassing its specific impulse.
Current rocket propellants include anhydrous hydrazine, monomethylhydrazine, and unsymmetrical dimethylhydrazine, predominantly used as bipropellant fuels in rockets like Titan or Ariane.
Anhydrous hydrazine also serves as a monopropellant for satellites and spacecraft. Its decomposition over a catalyst produces a mixture of gases.
Catalysts, often based on iridium or ruthenium deposited on alumina, are categorized as spontaneous (working at room temperature) or nonspontaneous (working above 100 °C).
3.8. Fuel Cells
Extensive research has been conducted on fuel cells that utilize the oxidation of hydrazine with either oxygen or hydrogen peroxide. However, their use is primarily limited to military applications due to the cost of hydrazine hydrate.
Reference
- Hydrazine; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_177


