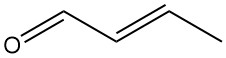
Crotonaldehyde [4170-30-3], also known as 2-butenal, is a colorless liquid with a pungent odor and strong lacrimatory properties. It has a chemical formula of CH3CH=CHCHO and exists as two stereoisomers: cis-crotonaldehyde [15798-64-8] and trans-crotonaldehyde [123-73-9].
Commercially available crotonaldehyde contains a mixture of both isomers, with the trans isomer being the dominant form (>95%) due to its greater thermodynamic stability.
Crotonaldehyde is found naturally in various plants and plant products, including French beans, rapeseed oil, and soybean oil.
Table of Contents
| Property | Value |
|---|---|
| Molecular Mass | 70.09 g/mol |
| Boiling Point | 102.2 °C |
| Melting Point | -76 °C |
| Density (20°C) | 0.852 g/cm3 |
| Refractive Index (n20D) | 1.438 |
| Heat of Vaporization | 515 J/g |
| Crotonaldehyde-Water Azeotrope | 24.8 wt% H2O |
| Azeotrope Boiling Point | 84 °C |
| Solubility in Water (20°C) | 181 g/L |
| Solubility of Water in Crotonaldehyde (20°C) | 9.5 g/100 g |
| Vapor Pressure (at 20 °C) | 4.3 kPa |
| Flash Point | 13 °C |
| Auto-ignition Temperature | 165 °C |
| Explosion Limits in Air |
Lower: 2.1 vol% Upper: 15.5 vol% |
2. Chemical Reactions of Crotonaldehyde
Crotonaldehyde is highly reactive due to the presence of both a carbonyl group and a carbon-carbon double bond in its structure. Contamination with strong acids or alkalis readily initiates exothermic condensation reactions.
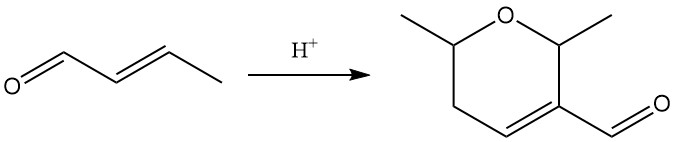
Exposure to strong acids catalyzes the dimerization of crotonaldehyde to dicrotonaldehyde (5,6-dihydro-2,6-dimethyl-2H-pyran-3-carboxaldehyde).
The primary reactions of crotonaldehyde include hydrogenation, reduction, oxidation, and addition.
Both the carbonyl group and the C-C double bond can undergo reduction. Catalytic hydrogenation, typically using nickel or copper catalysts, offers the most economical approach.
Selective hydrogenation of the olefinic moiety at lower reaction temperatures and pressures was a process formerly employed for the production of n-butanal. Currently, hydroformylation of propene is the preferred method for butyraldehyde synthesis.
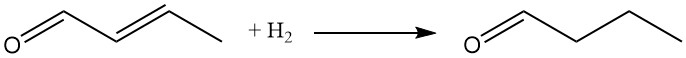
The hydrogenation of both functional groups produces n-butanol, commonly produced today via n-butyraldehyde hydrogenation.
Selective catalytic hydrogenation at the carbonyl group leads to crotyl alcohol (CH3CH=CHCH2OH).

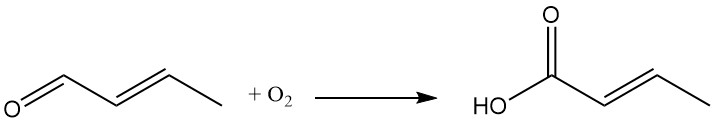
Crotonaldehyde oxidation results in the formation of crotonic acid.
The addition of various molecules across the double bond of crotonaldehyde makes it a valuable chemical intermediate. For example, the addition of alcohols or thiols to the olefinic double bond, catalyzed by a basic catalyst, is important for the synthesis of 3-alkoxybutyraldehydes.
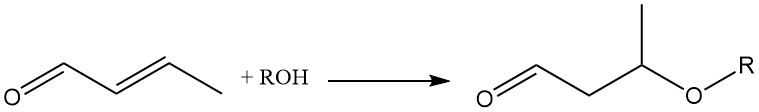
The addition of methanol to crotonaldehyde forms the intermediary 3-methoxybutyraldehyde, which undergoes consecutive hydrogenation and esterification to produce specialty solvents like 3-methoxybutanol (1) and 3-methoxybutyl acetate (2).

Crotonaldehyde can also be used as a dienophile in Diels-Alder reactions.
3. Production of Crotonaldehyde
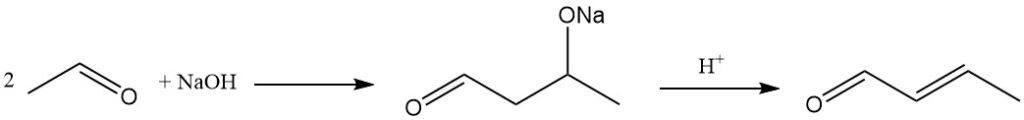
The dominant industrial method for crotonaldehyde production is the aldol condensation of acetaldehyde, followed by dehydration of the resulting aldol and subsequent distillation. This process yields a product with around 99% purity.
The aldolization reaction can be catalyzed by a wide range of basic catalysts, including alkali or alkaline earth metal catalysts, ammonium salts, zeolites, molecular sieves, and clay-like materials.
Acetic acid, mineral acids, or acidic cation-exchange resins are used as catalysts for the dehydration step that converts aldol to crotonaldehyde.
Celanese Chemicals and Daicel are major commercial producers of crotonaldehyde. A simplified continuous crotonaldehyde production unit is illustrated in Figure 1.
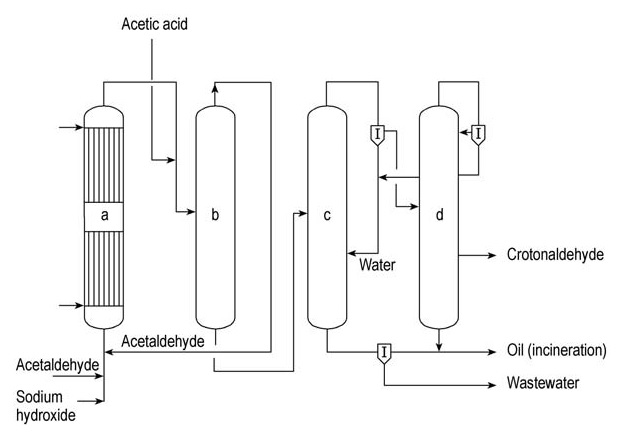
a) Aldol reactor; b) Acetaldehyde stripping column; c) Azeotrope distillation column; d) Rectification column
In this process, acetaldehyde undergoes aldolization in a water-cooled aldol reactor (a) using an aqueous solution of sodium hydroxide as the catalyst. The product is then neutralized with acetic acid before being fed into a stripping column (b).
Unreacted acetaldehyde is recovered at the top of this column and recycled back to the aldol reactor (a). The bottom product of column (b), the intermediate aldol, is fed into the azeotrope distillation column (c). Here, the aldol undergoes dehydration, and the crotonaldehyde-water azeotrope is distilled.
This azeotrope is subsequently separated into water and an aqueous crotonaldehyde phase containing approximately 10% water. The water is recycled back to the azeotrope column (c), exiting as the bottom product.
The aqueous crotonaldehyde stream is then fed into the rectification column (d), where another crotonaldehyde-water azeotrope forms and exits overhead. This azeotrope is separated into water, which is recycled to the azeotrope column (c), and aqueous crotonaldehyde, which is recycled back into the rectification column (d).
Pure crotonaldehyde is obtained as a side stream from this column.
Heavy fractions are removed from the bottom of the column (d) and combined with organic residues from the azeotrope column (c) before being incinerated. Wastewater generated from column (c) is treated in a dedicated wastewater purification plant.
Alternative production methods
While the aldol condensation of acetaldehyde remains the dominant industrial method, alternative technologies have been explored. One method is the oxidation of butadiene to its monoepoxide, followed by isomerization to crotonaldehyde.
This process has some similarities to the Wacker-Hoechst process for acetaldehyde production from ethylene. However, the use of paraldol as an alternative to aldol for cosmetic-grade 1,3-butylene glycol production appears to be more promising than its application in crotonaldehyde synthesis.
Other methods, such as the catalytic oxidation of olefins, dehydrogenation of allyl alcohol with amines, vapor phase hydration of acetylene, and enzymatic oxidation of 2-butene, have not achieved practical imortance for crotonaldehyde production.
Crotonaldehyde can also be formed as a byproduct during the synthesis of 1,3-butylene glycol and in acetic acid production via methanol carbonylation. Additionally, incomplete combustion processes, such as those occurring in automobile exhausts, generate crotonaldehyde as a component of the exhaust gas.
4. Uses of Crotonaldehyde
Crotonaldehyde is used as a precursor in the synthesis of various industrial products.
Large quantities of crotonaldehyde are used in the production of sorbic acid, a common food preservative. The synthesis involves condensation of crotonaldehyde with ketene in the presence of organic zinc salts, followed by depolymerization of the resulting polyester, either thermally or using a mineral acid.
α-Tocopherol, one of the eight naturally occurring components with vitamin E activity, is synthesized industrially via the condensation of phytol with 2,3,6-trimethylhydroquinone which is formed from crotonaldehyde and diethyl ketone through a four-step process.
3-Methoxybutanol is produced by the addition of methanol to crotonaldehyde followed by hydrogenation. This product and its acetate ester are used as specialty solvents, particularly valued for their ability to control viscosity, drying behavior, and gloss in lacquers and varnishes.
Crotonaldehyde is a precursor for a broad range of chemical intermediates. A primary example is crotonic acid, obtained by the oxidation of crotonaldehyde. These crotonaldehyde-derived intermediates are used in various sectors, including:
- Pharmaceuticals and biocompatible/medical products
- Agrochemicals
- Resins, polymeric thickeners
- Paints and coatings
- Dyestuffs
- Rubbers and rubber antioxidants
- Gelatin hardening
- Adhesives
- Leather tanning and sizing (leather and paper)
- Metal brighteners
- Lubricants
- Corrosion inhibitors
5. Toxicology of Crotonaldehyde
Crotonaldehyde poses a significant health risk due to its potent irritant properties. Here’s a breakdown of its effects:
Human Health Effects
- Crotonaldehyde irritates the eyes, skin, and respiratory tract. Studies suggest varying thresholds for irritation, ranging from 0.035 ppm to 0.56 ppm for odor detection and irritation, respectively.
- Inhalation can cause burning sensations in the nose and respiratory tract, tearing, coughing, bronchoconstriction, and potentially pulmonary edema or deep lung damage.
- Crotonaldehyde is present in tobacco smoke, engine exhaust, and wood burning. It also occurs naturally in some foods.
Occupational Exposure Limits
In the absence of specific occupational exposure levels, guidelines like AEGLs (acute exposure guideline levels) and ERPGs (emergency response planning guidelines) can be used for risk assessment during accidents or emergencies.
Toxicological Data
- Acute Toxicity:
- Oral LD50 (rat): 174 mg/kg
- Inhalation LC50 (rat, male, 4h): 336 mg/m3
- Dermal LD50 (guinea pig): 26 mg/kg
- Genotoxicity: While crotonaldehyde forms adducts in vitro (in a lab setting), evidence for in vivo mutagenicity is less clear.
- Crotonaldehyde is classified as mutagenic according to CLP Regulation (EC) No. 1272/2008.
Effects on Animals and Microorganisms
- Animals:
- Fish are the most sensitive species to crotonaldehyde, with a 96-hour LC50 (lethal concentration for 50% of the population) of 0.65 mg/L for rainbow trout.
- Crotonaldehyde irritates the respiratory tract in various animals, with rats being the most sensitive (4-hour inhalation LC50 of 120 ppm).
- Microorganisms are less susceptible than animals, with Pseudomonas putida showing an 18-hour EC10 (effect concentration for 10% of the population) of 10.4 mg/L.
Crotonaldehyde is classified as a marine pollutant despite its high biodegradability (> 83%).
References
- Crotonaldehyde and Crotonic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a08_083.pub2
- CROTONALDEHYDE; IARC MONOGRAPHS VOLUME 63. – https://publications.iarc.fr/81


