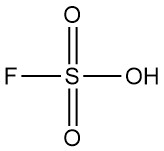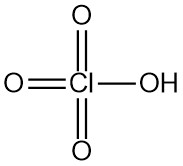
Perchloric acid is a strong mineral acid with the chemical formula HClO4. It is a colorless, odorless liquid that is usually found in aqueous solutions. Perchloric acid is one of the strongest Brønsted-Lowry acids.
Table of Contents
1. Properties of Perchloric Acid
1.1. Physical Properties of Perchloric Acid
Anhydrous perchloric acid can be produced by distillation at reduced pressure from a mixture containing 1 part of 20% HClO4 and 4 parts of 20% oleum.
It is a colorless, strongly hygroscopic liquid with a molecular mass of 100.5 g/mol. It melts at -102 °C and decomposes at 75 °C at atmospheric pressure.
The density and vapor pressure of perchloric acid at different temperatures are detailed in Table 1.
| Temperature, °C | Density, g/cm3 | Vapor Pressure, kPa |
|---|---|---|
| 0 | 1.808 | 1.546 |
| 10 | 1.789 | 2.506 |
| 20 | 1.770 | 3.913 |
| 25 | 1.761 | 4.826 |
Perchloric acid is miscible with water in all proportions and forms different hydrates (HClO4·n H2O, with n = 1, 2, 2.5, 3, and 3.5).
Boiling points and densities of aqueous solutions are presented in Table 2.
| Concentration of HClO4, wt % | Boiling Point, °C | Density at 25 °C, g/cm3 |
|---|---|---|
| 24.34 | 105.8 | 1.154 |
| 38.9 | 114.8 | 1.280 |
| 50.67 | 132.4 | 1.4058 |
| 56.65 | 148.0 | 1.4799 |
| 61.2 | 162.3 | 1.5413 |
| 65.20 | 189.2 | 1.5993 |
| 70.06 | 198.7 | 1.6748 |
| 72.5 | 203 | 1.7150 |
1.2. Chemical Properties of Perchloric Acid
Anhydrous perchloric acid is a very strong oxidizing agent. While some metals (nickel, copper, silver, and gold) show minimal oxidation at room temperature, platinum remains unaffected but catalyzes acid decomposition. Perchloric acid reacts violently with combustible materials like paper, charcoal, ethanol, acetic anhydride, and gelatin, forming explosive mixtures.
Aqueous solutions offer greater stability compared to the anhydrous form. The azeotropic mixture (72.5 wt% HClO4) decomposes only above its boiling point in the absence of oxidizable materials.
Concentrated perchloric acid solutions are strong oxidizing agents, particularly at elevated temperatures. These solutions form explosive mixtures with organic compounds that can detonate upon heating, percussion, or exposure to sparks or flames.
Perchloric acid is a strong Brønsted-Lowry acid, reacting in aqueous solutions with metals, metal oxides/hydroxides, and salts of volatile acids to form corresponding perchlorates.
Certain oxides (e.g., CuO) can catalyze perchloric acid decomposition through a series of reactions yielding chlorine, oxygen, and water as final products.
2. Production of Perchloric Acid
Perchloric acid is produced by chemical and electrochemical methods, both in laboratories and commercially.
2.1. Chemical methods
In 1816, Von Stadion reacted potassium perchlorate with sulfuric acid under vacuum distillation to produce perchloric acid.
In 1830, Serullas obtained perchloric acid by decomposing aqueous chloric acid with heating, and in 1831, he successfully produced insoluble potassium fluorosilicate and perchloric acid by reacting potassium perchlorate with hydrofluosilicic acid.
2 KClO4 + H2SiF6 → K2SiF6 + 2 HClO4
In 1839, Henry transformed barium perchlorate to barium sulfate and perchloric acid solution using sulfuric acid. A similar method uses hydrochloric acid instead of sulfuric acid.
Later in the 19th century, Willard oxidized ammonium perchlorate with a mixture of nitric acid and hydrochloric acid,to yield perchloric acid, chlorine, nitrous oxide, and water. The dilute perchloric acid solution is concentrated by boiling.
34 NH4ClO4 + 36 HNO3 + 8 HCl → 34 HClO4 + 4 Cl2 + 35 N2O + 73 H2O
In the modern chemical method, Pernert, based on the work of Kreider & Mathers, has produced perchloric acid by reacting sodium perchlorate with concentrated hydrochloric acid. This process was successfully commercialized.
NaClO4 + HCl → NaCl + HClO4
2.2. Electrochemical methods
A continuous process developed in Germany to produce perchloric acid uses the anodic oxidation of chlorine gas dissolved in chilled concentrated perchloric acid (40%).
Cl2 + 8 H2O + 14 e– → 2 HClO4 + 14 H+
Several studies explored the electrochemical preparation of perchloric acid by anodic oxidation of hydrochloric acid at low temperatures (20 to -20 °C) using platinum electrodes and specific HCl concentrations (0.1–0.5 M) to achieve ~60% perchloric acid solution.
A three-compartment cell with a platinum anode and an iron cathode has been developed for the electrochemical production of perchloric acid from sodium chlorate. The process involves the oxidation of chlorate at the anode to form perchloric acid, followed by concentration and distillation steps for purification. Optimal conditions are low temperatures below 35 °C and an anode current density of 3.5 A/dm².
The commercial process for producing high-purity perchloric acid uses a 5000-ampere cell with platinum anodes and horizontally placed silver sheet cathodes within PVC frames. This process employs a current density of 20–25 A/dm² and a cell voltage of 4.4 V at temperatures ranging from -5 to 3 °C.
The chlorine concentration at the entry point is 3 g/L. The produced perchloric acid undergoes distillation to remove residual chlorine and HCl and achieve high purity. Platinum consumption is minimal, and the energy consumption per ton of 70% HClO4 is 9600 kWh.
This process allows for the direct production of different perchlorates by the direct conversion of perchloric acid without the need for the sodium chlorate process.
Anhydrous perchloric acid is produced by vacuum distillation of a mixture of concentrated perchloric acid (72%) and fuming sulfuric acid (20%) in a 1:4 ratio. The product is then recovered by cooling to a low temperature of -78 °C using dry ice.
3. Uses of Perchloric Acid
Commercially available perchloric acid is primarily an azeotropic aqueous solution containing 72.5 wt% HClO4, which offers a balance between chemical stability and oxidizing power, allowing controlled high-energy oxidations.
Perchloric acid is primarily used as a precursor for the production of high-purity ammonium perchlorate, a key component in explosives and solid propellants for rockets and missiles, which has led to an increase in perchloric acid production in recent times.
It finds application in analytical chemistry for the determination of metal elements present in oxidizable materials, particularly organic compounds.
Other applications of perchloric acid include:
- As a catalyst for the acetylation of cellulose and glucose, used in the preparation of cellulose fibers.
- In the polymerization of phenols and styrene.
- In metal processing, perchloric acid is used for electropolishing, machining, and thinning of metal parts, films, and alloys, as well as pickling and passivation of iron, steel, and other metals.
- As an electrolyte in the anodization of metals, which creates a protective oxide layer.
- As a dehydration molecule.
- In oxidation reactions, particularly for the determination of chromium in steel samples.
Reference
- Chlorine Oxides and Chlorine Oxygen Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_483.pub2