
Acetone, also known as 2-propanone, is a colorless, flammable liquid with a pungent odor and low viscosity. It is the simplest ketone, with the formula C3H6O. Acetone is miscible with water, meaning it mixes readily with water in any proportion.
It is primarily used as a solvent and intermediate in the synthesis of various chemicals, such as bisphenol A, methyl methacrylate, aldol chemicals (diacetone alcohol, mesityl oxide, and methyl isobutyl ketone), and other products.
Historically, acetone was produced commercially via the dry distillation of calcium acetate up until the early 1900s.
Acetone is also present in nature, such as in trees and plantsand It is also metabolically produced within the human body, mainly from the breakdown of fat. Furthermore, acetone is readily biodegradable.
Table of Contents
1. Production of acetone
At present, the cumene process stands as the most significant means for the manufacture of acetone, accounting for over 6 million tons of production globally each year.
Other production methods contribute less than 5% to the worldwide capacity.
1.1. Production of Acetone by Cumene Process
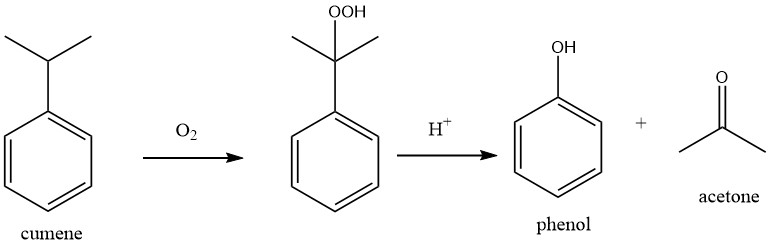
The production of acetone from benzene and propene involves a two-step process.
In the first step, Friedel-Crafts alkylation is utilized to produce cumene from benzene and propene in the cumene process.
During this process, low-boiling hydrocarbons, such as propane, are separated from the propene feedstock in the cumene distillation unit. The high-boiling components, mainly polyisopropylbenzenes, are separated and collected as a residue.
The benzene and propene feedstocks generate a small amount of water as impurities.
In the second step of the process, known as the Hock process, cumene is oxidized with atmospheric oxygen to produce cumene hydroperoxide (CHP). Using a strong mineral acid as a catalyst, CHP is then cleaved to yield phenol and acetone.
During the distillation process, low-boiling components, primarily ketones from acetone, are separated, while the high-boiling components formed in the oxidation and cleavage unit are collected as a residue.
In the phenol-acetone process, a certain amount of process water is generated and must be treated in a biological wastewater treatment plant.
The overall reaction of the process can be described as a dual oxidation, wherein benzene is oxidized to phenol, and propene is oxidized to acetone.
1.2. Production of Acetone by Dehydrogenation of 2-Propanol

The dehydrogenation of 2-propanol (IPA), which is formed through the hydration of propene, represents an alternative route to produce acetone. The dehydrogenation reaction is known to be endothermic.
This process was responsible for approximately 50-60% of the total acetone production in the USA during the 1970s.
However, presently, the cumene oxidation process with acetone as a co-product has become the major source of acetone production worldwide.
Several catalysts have been reported in the literature for the dehydrogenation of 2-propanol.
Propene is the primary by-product, which is formed by the dehydration of the corresponding alcohol. In addition to propene, aldehydes and mesityl oxide are also formed.
The dehydrogenation product, which contains aldehydes, mesityl oxide, water, 2-propanol, and acetone, is subjected to a washing step with 35% aqueous caustic soda to remove the aldehydes. This is followed by distillation in several steps to remove light boilers and acetone from the alcohol and water.
1.3. Production of Acetone by Propene Oxidation

The Wacker-Hoechst process offers an elegant method for the production of acetone. This process involves the oxidation of propene using air or oxygen as the oxidant, at a temperature range of 110-120°C and a pressure of 10-14 bar.
A catalyst system consisting of PdCl2 and CuCl2 is used in the process.
The PdCl2 acts as the catalyst, while the CuCl2 reoxidizes Pd° to the bivalent state. The copper(I) chloride can be easily oxidized to copper(II) chloride using oxygen.
The selectivity of this process for acetone is approximately 92%, with propionaldehyde being formed as a byproduct, along with chlorinated compounds such as monochloracetone and 1,1-dichloroacetone.
Two different processes for direct oxidation are possible, similar to the acetaldehyde process.
In the first process, the catalyst is treated with propene and oxygen in a single stage.
In the second process, the catalyst is treated with propene in the first stage and then regenerated in the second stage using oxygen.
The two-stage process is preferred as a propene-propane mixture can be used as a feed. Propane is inert to oxidation and does not participate in the reaction.
2. Chemical Reactions of acetone
Pure acetone is highly sensitive to powerful oxidizing agents, such as hydrogen peroxide and organic peroxides.
When exposed to dilute acid and hydrogen peroxide, acetone peroxide trimer is formed.
When acetone reacts with strong reducing agents, it generates heat and may cause explosions.
Strong alkali also triggers heat production when reacting with acetone.
Additionally, acetone mixed with chloroform in the presence of alkali can cause a violent reaction.
The reactivity of acetone is governed by the CO bond. The oxygen atom tends to attract electrons of the double bond, as shown in the mesomeric structure of the carbonyl group. As a result, the most common carbonyl reaction mechanism is nucleophilic attack on the C atom of the carbonyl group.
Protonation of the oxygen atom frequently accompanies nucleophilic attack.
The reaction of acetone with HCN in the presence of a base such as KOH leads to the formation of acetone cyanohydrin, with the cyanide ion, derived from HCN and KOH, responsible for the nucleophilic attack.
Acids catalyze the addition of weak nucleophiles.
The protonation of the oxygen atom of the carbonyl group creates a carbocation that can be more readily attacked by the nucleophile. An example of such a reaction is the formation of a hemiketal or a ketal from the reaction of an alcohol with acetone.
Various nucleophilic reactions with acetone have been reported in the literature, such as the formation of tertiary alcohols by reacting with Grignard reagents (RmgX), 2-propanol with LiAlH4 or NaBH4, and imines with primary amines, oxime with hydroxylamine, and phenylhydrazone with hydrazine.
The α-hydrogen atom to the carbonyl group in acetone is acidic. The carbonyl group’s presence enhances electron polarization, resulting in a more electropositive hydrogen atom. The resulting anion is stabilized by mesomeric dislocation of the negative charge between the carbon atom and the carbonyl oxygen atom.
The base-catalyzed aldol reaction of two acetone molecules in the presence of alkali produces diacetone alcohol.
3. Uses of acetone
Acetone is used as a chemical intermediate in the production of several chemicals, including methyl methacrylate (MMA), bisphenol A, and aldol compounds such as diacetone alcohol (DAA), mesityl oxide (MOX), and methyl isobutyl ketone (MIBK).
Its primary use in the chemical industry is to produce acetone cyanohydrin.
Aside from its use as a chemical intermediate, acetone is a versatile solvent with numerous applications. The global annual production of acetone is estimated to be over 6 million tons.
In 2010, direct solvent applications accounted for 29% of acetone use, followed by acetone cyanohydrin/MMA at 24% and bisphenol A at 22%
Reference
- Acetone; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_079.pub4

