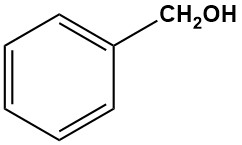
What is benzyl alcohol?
Benzyl alcohol is the simplest and most important industrial aromatic alcohol, with the chemical formula C6H5CH2OH. It is a colorless liquid at room temperature with a faint, pleasant odor that is slightly soluble in water but miscible with many organic solvents.
Benzyl alcohol was first prepared from bitter almond oil by Liebig and Wohler in 1832. Cannizzaro determined its structure in 1853 using his namesake reaction, in which benzaldehyde is disproportionately converted into benzoic acid and benzyl alcohol by alkali.
Benzyl alcohol is found in nature both in free form and as esters, such as acetic, benzoic, salicylic, and cinnamic acid esters. It is found in Peru and Tolu balsams, hyacinth and wallflower flower oils, ylang-ylang oil, and other essential oils. It also occurs as a glucoside in maize.
Table of Contents
1. Physical Properties of Benzyl Alcohol
Benzyl alcohol is a colorless liquid with a faint aromatic odor and a mildly irritating effect on the mucous membranes. It is missible with many organic solvants.
The following table present some of the physical properties of benzyl alcohol:
| Property | Value |
|---|---|
| Molecular formula | C7H8O |
| Molecular weight | 108.14 g/mol |
| Boiling point | 205.4 °C at 101.3 kPa |
| Melting point | -15.4 °C |
| Refractive index | 1.5400 |
| Density | 1.061 g/cm3 at 0 °C |
| Specific heat | 1972 J/kg·K at 20 °C |
| Heat of fusion | 82.9 J/g |
| Heat of vaporization | 467.0 J/g at 205.4 °C |
| Standard combustion enthalpy | 34.58 kJ/g |
| Flash point | 101 °C |
| Autoignition temperature | 435 °C |
| Lower explosive limit | 1.3 vol % at 170 °C and 101.3 kPa |
| Upper explosive limit | 13.0 vol % |
| Solubility in water | 4.0 g/100 g at 20 °C |
| Solubility of water in benzyl alcohol | 5.1 g/100 g at 20 °C |
| Dynamic viscosity | 5.584×10-3 Pa·s at 20 °C |
| Surface tension | 39.96×10-3 N/m at 20 °C |
| Dipole moment | 5.571×10-30 C·m (1.67 D) |
| Relative dielectric constant | 11.92 at 30 °C |
| Relative dielectric constant | 9.81 at 60 °C |
2. Chemical Reactions of Benzyl Alcohol
Benzyl alcohol’s unique properties are primarily due to its hydroxyl group, which behaves like those of aliphatic alcohols. However, its proximity to the aromatic ring makes it more reactive. Benzyl alcohol is less acidic than isomeric cresols, so it doesn’t fully dissolve in aqueous alkalis.
When heated with dehydrating agents like aluminum oxide, benzyl alcohol produces a variety of compounds, including dibenzyl ether, toluene, and benzaldehyde. Dibenzyl ether can also be formed by reacting benzyl alcohol with alkyl halides in the presence of bis(acetylacetonato) nickel as a catalyst.

Oxidation of benzyl alcohol gives benzaldehyde or benzoic acid, depending on the oxidizing agent and the reaction conditions. For example, nitric acid oxidizes benzyl alcohol to benzaldehyde, while solid sodium permanganate monohydrate oxidizes it to benzoic acid.
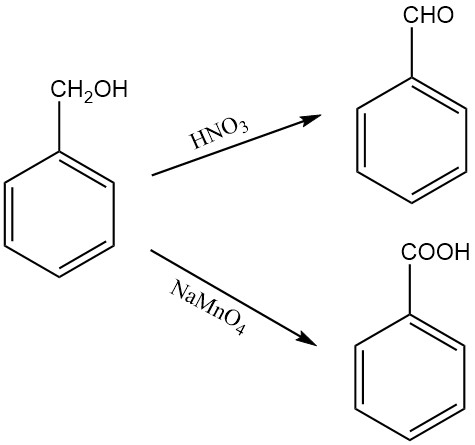
Benzyl alcohol can also be oxidized to benzaldehyde under Oppenauer oxidation conditions, using furfural as the hydrogen acceptor. Exposure to air can gradually oxidize benzyl alcohol to benzaldehyde.
Dehydrogenation of benzyl alcohol in the gas phase, using copper or noble metal catalysts, also produces benzaldehyde as the main product.
Hydrogenation of benzyl alcohol under different conditions can produce a variety of products, including toluene, benzene, methylcyclohexane, cyclohexane, and hydroxymethylcyclohexane. Benzyl alcohol also reacts with hydrogen halides to form the corresponding benzyl halides.

In addition, benzyl alcohol can be converted to benzoyl chloride when chlorinating the side chains of aromatic compounds. It can also be converted to various alkylamines when reacted with ammonia or amines.
Friedel-Crafts catalysts can be used to alkylate benzyl alcohol. For example, reacting benzyl alcohol with benzene gives diphenylmethane, while reacting it with phenol gives a mixture of 2- and 4-benzylphenols.
Benzyl alcohol reacts with organic and inorganic acids, acid halides, or acid anhydrides to form esters. Acid-catalyzed reactions with aldehydes give acetals. In addition, reacting benzyl alcohol with carbon monoxide in the presence of carbonyl catalysts gives phenylacetic acid.
Treating benzyl alcohol with certain catalysts, such as anhydrous aluminum chloride or zinc chloride, removes water to produce resinous products. Even small amounts of hydrogen bromide and iron(II) can initiate an exothermic polycondensation reaction of benzyl alcohol at temperatures above 150 °C.
This reaction can increase the temperature to 240 °C and cause a sudden pressure surge in a closed vessel. Therefore, it is recommended not to heat benzyl alcohol above 100 °C unless it is free of acidic impurities and dissolved iron.
3. Production of Benzyl Alcohol
There are only two industrially important methods for producing benzyl alcohol:
- Hydrolysis of benzyl chloride
- Hydrogenation of benzaldehyde
3.1. Production of Benzyl Alcohol by Hydrolysis of Benzyl Chloride

The hydrolysis of benzyl chloride is a reversible reaction that can be made almost quantitative in the presence of basic saponifying agents that neutralize the hydrochloric acid produced as a byproduct.
The reaction is typically carried out by heating benzyl chloride with an excess of an aqueous solution of an alkali or alkaline earth metal oxide, hydroxide, or carbonate. Soda is the preferred saponifying agent.
To produce benzyl alcohol on an industrial scale, the following procedure is typically used:
- 126.5 parts of benzyl chloride are added to 610 parts of boiling 10% soda solution with stirring.
- The reaction mixture is refluxed and stirred until carbon dioxide no longer escapes (this takes about 5-6 hours).
- The reaction mixture is cooled and the upper layer, consisting of crude benzyl alcohol, is removed.
- The sodium chloride solution below is extracted with organic solvant like benzene or toluene to recover any dissolved benzyl alcohol.
- The crude benzyl alcohol is purified by fractional distillation at reduced pressure to obtain pure product.
The yield of benzyl alcohol from this process is 67% and for dibenzyl ether is 8%.
To reduce the reaction time, the final residue of benzyl chloride can be hydrolyzed with sodium hydroxide solution after 3 hours of saponification with soda solution. This reduces the total saponification time to 4 hours.
The percentage of dibenzyl ether formed in the reaction can be reduced by using an inert solvent, such as benzene, toluene, or xylene, or by hydrolyzing the benzyl chloride in a continuous process.
In a new continuous process, benzyl chloride and the alkaline saponifying agent are reacted as counter-currents in an inert organic solvent in a flow reactor. The alcohol is extracted from the aqueous alkaline phase by an inert organic solvent in an extraction zone.
The crude benzyl alcohol is then washed with water in a washing zone. The flow reactor, extraction zone, and washing zone are integrated in a special apparatus. This process can be operated at low reaction temperatures (120-150 °C) and with a small stoichiometric excess of saponifying agent (5-25%).
Two-step processes for producing benzyl alcohol have also been developed, but these have not gained much commercial importance.
3.2. Production of Benzyl Alcohol by Hydrogenation and Reduction of Benzaldehyde
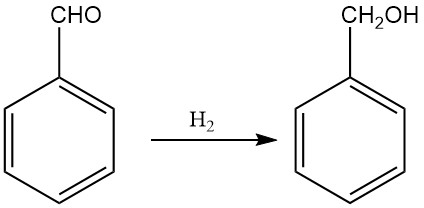
The industrial production of benzyl alcohol by hydrogenation of benzaldehyde became important because of the large amounts of benzaldehyde available as a byproduct of the Dow process for phenol production and the Snia Viscosa process for caprolactam production.
Depending on the reaction conditions, benzaldehyde can be hydrogenated to form a variety of products, including benzyl alcohol, toluene, hydroxymethylcyclohexane, and methylcyclohexane. However, high yields of benzyl alcohol can be obtained by adjusting the reaction conditions and catalysts.
Suitable catalysts for the hydrogenation of benzaldehyde include:
- Raney nickel doped with transition metals
- Nickel or platinum metals with phosphines or phosphine oxides
- Palladium combined with an organic nitrogen, alkali base, water, or another transition metal
When benzaldehyde is hydrogenated at temperatures of 70-200 °C and a hydrogen pressure of 1-4 MPa, high yields of benzyl alcohol are obtained in short reaction times.
A well-known continuous process uses a platinum-aluminum oxide-lithium oxide catalyst to efficiently and selectively hydrogenate benzaldehyde to benzyl alcohol.
Other methods using reducing agents such as stannane, sodium hydride, zinc, or microorganisms, have no industrial importance.
The Cannizzaro reaction is no longer used industrially to produce benzyl alcohol.
3.3. Production of Benzyl Alcohol by Oxidation of Toluene
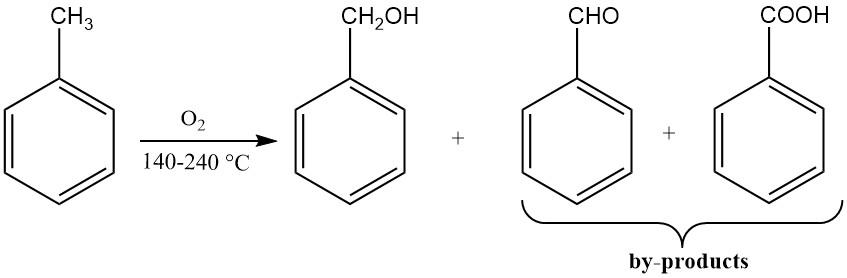
The catalytic oxidation of toluene yields low yields of benzyl alcohol because the reaction conditions favor further oxidation to benzaldehyde and benzoic acid. Most benzyl alcohol production processes using toluene as raw material isolate intermediate byproducts.
One process is to oxidize toluene with oxygen-depleted air at 170-220 °C and sufficient pressure to keep the reaction medium liquid. This reaction is carried out in the presence of stabilizers for organic hydroperoxides, such as sodium pyrophosphate or sodium fluoride.
If not more than 10% of the toluene is allowed to react, the main product is benzyl hydroperoxide. The subsequent decomposition of benzyl hydroperoxide at 165 °C in the presence of soluble cobalt salts yields benzyl alcohol.
Benzaldehyde and benzoic acid are formed as byproducts. Benzyl hydroperoxide can also be reduced to benzyl alcohol using alkali metal sulfites.
Another process is to oxidize toluene in air in the liquid phase in the presence of acids, acid chlorides, or acid anhydrides. This forms the benzyl esters of these acids, which can then be saponified to form benzyl alcohol.
For example, the oxidation of toluene in the presence of acetic anhydride at 140-240 °C and 1-3 MPa yields benzyl acetate. The oxidation is discontinued after 10% of the toluene has reacted. After saponifying the oxidation product, 350 g of benzyl alcohol, 55 g of benzaldehyde, and 67 g of benzoic acid can be obtained from 500 g of reacted toluene.
Phenolic impurities, such as cresols, are unavoidably present in the benzyl alcohol obtained by the oxidation of toluene. These impurities can be removed by washing the benzyl alcohol vapor with a countercurrent of alkali benzylate solution in a plate column or a packed column.
3.4. Other Manufacturing Processes
The hydrogenation of benzoic acid esters to form benzyl alcohol is an attractive method when benzoic acid esters are produced in large quantities, such as in the production of dimethyl terephthalate by the Witten process.
Copper catalysts are preferred for this reaction. Benzoic acid esters can be hydrogenated to benzyl alcohol using a copper catalyst supported on alkaline earth oxides or carbonates. Selectivity can be improved by adding chromium to the catalyst. The reaction is typically carried out at temperatures of 100-300 °C and pressures above 6 MPa.
Other catalysts for the hydrogenation of benzoic acid esters to benzyl alcohol include ruthenium, rhodium, platinum, and palladium, activated by alkali metal arenes, ketyls, or alkoxides. These catalysts allow for the reaction to be carried out under mild conditions with high selectivity.
Three-step processes for the production of benzyl alcohol from toluene typically involve the hydrogenation of methyl benzoate in the presence of a copper-chromium catalyst:
- Oxidation of toluene to benzoic acid
- Esterification with methanol
- Hydrogenation of methyl benzoate to benzyl alcohol
This process produces benzyl alcohol at a very low cost.
Other processes including hydrogenation or electrochemical reduction of benzoic acid, hydrolysis of benzylsulfonic acid, and decarboxylation of benzyl formate are not important for the industrial production of benzyl alcohol, but they may be used to produce derivatives that are substituted on the aromatic ring.
Benzyl alcohol can also be obtained from the benzyl benzoate that is produced as a byproduct of benzoic acid manufacture.
4. Uses of Benzyl Alcohol
Benzyl alcohol is a good solvent for surface coatings, resins, cellulose esters, ethers, alkyd resins, acrylic resins, and fats. It is also used in inks for ball-point pens, to improve the flow and gloss of surface coatings, and as an auxiliary in the dyeing of wool, polyamides, and polyesters.
Because it has a faint odor, it is used as a solvent and diluting agent in perfumes and flavors.
Benzyl alcohol is also used as a development accelerator in color photography.
It is used as a local anesthetic in pharmacy, and as an ingredient in ointments and other preparations due to its microbicidal effect.
Benzyl alcohol is a starting material for many benzyl esters, which are used as odorants, flavors, stabilizers for volatile perfumes, and plasticizers.
It is also used in the extractive distillation of m- and p-xylenes and m- and p-cresols.
Other uses of benzyl alcohol include:
- Solvent for cleaning and degreasing
- Fuel additive
- Personal care product ingredient (e.g., shampoo, conditioner, toothpaste)
- Pharmaceutical excipient (e.g., preservative, solubilizer)
- Agricultural chemical (e.g., miticide, insecticide)
5. Toxicology of Benzyl Alcohol
Benzyl alcohol is a versatile fragrance, preservative, and antimicrobial agent used in pharmaceuticals, soaps, detergents, cosmetics, and food products. It is considered “generally regarded as safe” (GRAS) by the U.S. Food and Drug Administration, but environmental or workplace exposure limits have not yet been established.
Benzyl alcohol is moderately toxic, with acute oral LD50 values of 1.2 g/kg in rats and 1.6 g/kg in mice. It can also be absorbed through the skin in toxic amounts, with a dermal LD50 in guinea pigs of less than 5 mL/kg.
Benzyl alcohol is not classified as a carcinogen, and there is no data on its teratogenic or reproductive effects. However, it is important to note that benzyl alcohol is highly toxic to newborn humans, with an estimated lethal dose significantly lower than in adults.
Benzyl alcohol is widely used as a preservative in intravenous drug solutions, but caution is required due to its high toxicity in newborns.
Benzyl alcohol is also severely toxic and irritating to the eyes, and can cause mild to moderate skin irritation. Some people may experience hypersensitivity reactions to benzyl alcohol, both topically and parenterally.
Symptoms of benzyl alcohol intoxication include vomiting, diarrhea, central nervous system depression, excitability, muscle paralysis, convulsions, respiratory distress, and collapse.
To reduce exposure to benzyl alcohol, it is important to use adequate ventilation systems, self-contained respiration equipment, protective eyewear, gloves, and clothing.
Reference
- Benzyl Alcohol; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_001