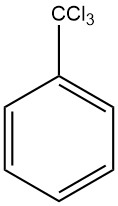
Benzotrichloride is the product resulting from exhaustive chlorination of the side chain of toluene also known as trichloromethylbenzene, α,α,α-trichlorotoluene and phenyl chloroform. It is a chemical compound with the formula C7H5Cl3.
The synthesis of benzotrichloride was initially accomplished in 1858 by L. SCHISCHKOFF and A. ROSING, employing the reaction between PCl5 and benzoyl chloride.
Today, due to it significant role as an important intermediate in the synthesis of acid chlorides (specifically benzoyl chloride), dyes, herbicides, pesticides, and various other products, benzotrichloride is produced on a large-scale.
Table of Contents
Physical Properties of benzotrichloride
Benzotrichloride is a colorless liquid with a pungent odor and is irritant to the eyes and mucous membranes. It generate fumes when exposed to moist air.
Ethanol, diethyl ether, and chloroform are good solvents for benzotrichloride. However, its solubility in water is limited, with only 0.05 g per liter at 5°C and 0.25 g per liter at 39°C. Notably, hydrolysis occurs during the process of dissolving in water.
The quantity of chlorine that can be dissolved in 100 grams of benzotrichloride is temperature-dependent. At 30°C, the solubility is 5.1 grams, while at 50°C it decreases to 3.4 grams, and at 100°C it further reduces to 1.3 grams.
The physical properties of benzotrichloride are the following:
- Molar mass = 195.48 g/mol
- Boiling point = 220.7 °C
- Melting point = -4.5 °C
- Density = 1.373 at 20 °C
- Refractive index = 1.558 at 20 °C
- Flash point = 108 °C
- Ignition temperature = 420 °C
Chemical Reactions of benzotrichloride
Benzoic acid is produced by either acid or alkaline hydrolysis of benzotrichloride, while partial hydrolysis produces benzoyl chloride.
When benzotrichloride reacts with carboxylic acids, it yields the corresponding acid chlorides as well as benzoyl chloride.
The condensation of benzotrichloride with benzene, in the presence of catalysts such as FeCl3, AlCl3, or ZnCl2, leads to the formation of diphenyl- and triphenylmethane.
Treatment of benzotrichloride with hydrofluoric acid or fluorides enables the replacement of all three chlorine atoms with fluorine.
By reacting benzotrichloride with anhydrous alcohols, ortho-esters of benzoic acid can be prepared.
Hydrolysis of benzotrichloride can be achieved by reacting with water.
Production of benzotrichloride
A method akin to the procedure employed for benzyl chloride can be used for the exhaustive chlorination of the side-chain of toluene, facilitating the production of benzotrichloride. Photochemical chlorination, in particular, is commonly employed for this purpose.
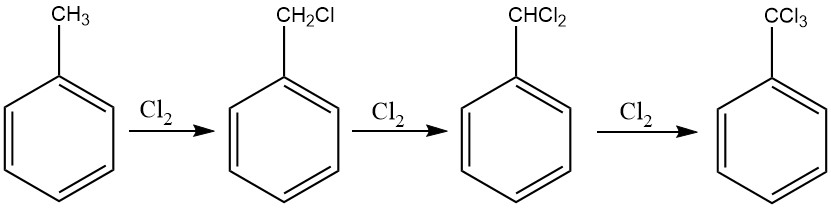
In continuous processes, it is advisable to implement a cascade of six to ten reactors to prevent the formation of ring-chlorinated derivatives. This approach enables control over the chlorine feed, ensuring that benzotrichloride with minimal amounts of benzal chloride is obtained.
Figure 1 depicts a continuously operated plant designed for the production of benzotrichloride. Fresh toluene is directed through the pre-chlorinator (R0) and subsequently enters the first reactor in the cascade of eight reactors. The reactors are charged with decreasing amounts of chlorine.
Typically, the last reactor in the cascade is purged with pure nitrogen to remove residual chlorine gas. For the purpose of waste gas removal (indicated by dashed lines), the reactors can be categorized into three groups.
Reactors R1-R3 receive precise chlorine metering, most of which is consumed during the reaction. The off-gas from reactors R4-R8 contains a higher concentration of chlorine due to the advanced chlorination of the material in these reactors. Consequently, this gas is recycled to reactors R1 and R2.
Likewise, the off-gas from reactors R1-R4 is introduced into the pre-chlorinator (R0), which contains the highest proportion of toluene, to eliminate any remaining traces of chlorine. The off-gas from reactor R0 is thus devoid of chlorine and is directed to the scrubber system to remove HCl.
By accurately controlling the feeds of chlorine and toluene, this technique achieves nearly complete conversion of toluene into benzotrichloride, generating waste gases predominantly composed of hydrogen chloride and nitrogen, with no chlorine present.

To enhance the yield and reaction rate, the exclusion of oxygen is imperative, and catalytic quantities of bromine, ammonium chloride, or a combination of phosphorus trichloride and bis(dimethylthiocarbamoyl) disulfide can be added.
Claimed to offer a high yield of highly pure products, the chlorination of methylbenzenes in the corresponding trichlorides as solvents presents an alternative method for the production of benzotrichloride.
Another manufacturing process for benzotrichloride involves chlorinating dibenzyl ether, a byproduct obtained during the conversion of benzyl chloride to benzyl alcohol. This chlorination process yields a mixture of benzotrichloride and benzoyl chloride, which can be subsequently processed to obtain pure benzoyl chloride.
This approach indirectly improves the economic viability of benzyl alcohol production.
Uses of benzotrichloride
The primary application of benzotrichloride lies in its utilization for the production of benzoyl chloride through partial hydrolysis with water or reaction with benzoic acid.
Benzotrichloride also finds smaller-scale applications in various industries. It is employed in the production of pharmaceuticals and agrochemicals, particularly after undergoing a transformation into benzotrifluoride. Additionally, it serves as a precursor for the synthesis of benzophenone-type UV stabilizers and dyes.
Toxicology of Benzotrichloride
Benzotrichloride is a toxic chemical that can cause irritation to the skin, eyes, and respiratory tract. It is also a probable carcinogen to humans.
The acute oral toxicity (LD50) of benzotrichloride is 2188 mg/kg in male rats and 1590 mg/kg in female rats. This means that 50% of rats will die if they ingest 2188 mg/kg or 1590 mg/kg of benzotrichloride, respectively.
The LC50 values for benzotrichloride are greater than 600 mg/m³ in male rats and approximately 500 mg/m³ in female rats after a 4-hour exposure period. This means that 50% of rats will die if they are exposed to benzotrichloride vapor at a concentration of greater than 600 mg/m³ or approximately 500 mg/m³, respectively, for 4 hours.
Benzotrichloride has also been shown to be mutagenic in bacterial test systems. This means that it can cause changes in DNA that can lead to cancer.
Inhalation exposure of female mice to benzotrichloride has resulted in both benign and malignant lung and skin tumors. In a mouse lung tumor study, intraperitoneal injection of benzotrichloride increased the incidence of lung adenomas.
Furthermore, oral administration of benzotrichloride to 40 female mice over 25 weeks induced neoplasms in the forestomach, lung, and thymic gland at the highest dose.
These findings suggest that benzotrichloride is a potential carcinogen to humans. It is important to avoid exposure to this chemical by using appropriate safety precautions when handling it.
Reference
- Benzyl Chloride and Other Side-Chain-Chlorinated Aromatic Hydrocarbons; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.o04_o01.pub2

