
Solid glycolic acid is the simplest α-hydroxycarboxylic acid with the formula C2H4O3. It forms colorless, monoclinic, prismatic crystals. This acid exhibits high solubility in various solvents, including water, methanol, ethanol, acetone, and ethyl acetate.
The volatility of glycolic acid is limited, with minimal tendency to evaporate when exposed to heat. Consequently, it cannot be effectively distilled under vacuum conditions.
Efforts to distill it often result in self-esterification, accompanied by water loss, leading to the formation of di- and polyglycolides.
Table of Contents
Physical Properties of Glycolic acid
- Molar mass = 76 g/mol
- melting point = 78-80 °C
- boiling point = 100 °C (decompose)
- Density at 25 °C = 1.49
- Refractive index (20 °C) = 1.423
- pKa = 3.81 at 25 °C
Chemical Reactions of Glycolic acid
Glycolic acid exhibits the ability to undergo dimerization by eliminating water, resulting in the formation of cyclic diester known as 1,4-dioxane-2,5-dione. These diesters are commonly referred to as lactides due to their initial discovery during studies involving lactic acid.

Polymerization of glycolic acid through polycondensation gives Polyglycolide or poly(glycolic acid) (PGA), which is a biodegradable and thermoplastic polymer. This type of esterification, known as estolide formation, is not limited to α-hydroxycarboxylic acids.

Another important characteristic of glycolic acid is that the proximity of its two functional groups weakens the intervening C-C bond(s). Consequently, treatment of such compounds with sulfuric acid leads to the elimination of formic acid. Upon exposure to concentrated sulfuric acid, formic acid decomposes, producing carbon monoxide and water.
This elimination reaction occurs with high efficiency, making it a valuable tool for the quantitative determination of glycolic acid.
Oxidative cleavage is also feasible for glycolic acid. For instance, when treated with hydrogen peroxide in the presence of iron(II) ions, glycolic acid easily undergoes carbon dioxide elimination.
Glycolic acid can be catalytically dehydrogenated to glyoxylic acid in the vapor phase.
When glycolic acid is reacted with PCl3, it forms chloroacetyl chloride (ClCH2COCl).
Production of Glycolic acid
Glycolic acid is commonly synthesized by the hydrolysis of molten monochloroacetic acid using a 50% aqueous solution of sodium hydroxide at temperatures ranging from 90 to 130 °C.
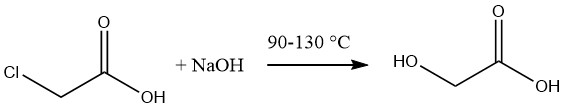
The resulting solution of glycolic acid has an approximate concentration of 60%, accompanied by a sodium chloride content of 12-14%. The elimination of the salt can be achieved through evaporation followed by extraction of the acid using acetone.
Alternative approaches have been explored wherein the hydrolysis is conducted with acid catalysts at higher temperatures of 150-200 °C, employing water or pressurized steam.
In this process, hydrogen chloride is formed as a byproduct instead of sodium chloride, which can be separated through distillation. However, this method necessitates the utilization of substantial amounts of water, which poses a significant drawback.
In the United States, commercial production of glycolic acid (by Du Pont) involves the treatment of formaldehyde or trioxymethylene with carbon monoxide and water in the presence of acid catalysts under pressures exceeding 30 MPa.

Another method, previously employed by Degussa, entails the electrolytic reduction of oxalic acid.
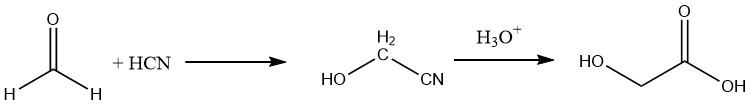
Furthermore, glycolic acid can be prepared with a yield of approximately 90% by hydrolyzing the corresponding nitrile, which is obtained by reacting formaldehyde with hydrocyanic acid.
A recent study show that glycolic acid can be produced by selective oxidation process of ethylene glycol (EG) using a highly efficient bimetallic PtMn/MCM-41 nanocatalysts.
Uses of Glycolic acid
Glycolic acid is commercially available in the form of aqueous solutions, with concentrations of 57% (Hoechst) or 70% (Du Pont). The global annual consumption of these solutions is approximately 2000-3000 tons.
In various industries, glycolic acid finds application in textile dyeing, printing, and creaseproofing. Its ability to form chelates with calcium(II) ions makes it particularly suitable for masking deliming in the leather industry. It is also utilized in alum and chrome mordants, as well as in fur-processing operations.
Due to its low corrosive nature and bactericidal properties, glycolic acid is commonly incorporated into acidic cleansing agents. It is especially effective in cleaning milk containers, milk-processing equipment, drinking fountains, and for removing rust and scale in heat exchangers and pipelines.
Glycolic acid exhibits inhibitory effects on the growth of iron-oxidizing bacteria. Its use eliminates the need for simultaneous addition of chelating agents and bactericides.
The complexing properties of glycolic acid also make it suitable for applications such as copper polishes, etching agent for lithographic plates, and in the formulation of electropolishing and galvanizing baths.
Derivatives of Glycolic acid
Methyl glycolate and ethyl glycolate are two glycolic acid esters commonly utilized as starting materials for the laboratory synthesis of pure glycolic acid. In the past, they were also employed as solvents for resins and nitro- or acetylcellulose. Aside from these esters, only carboxymethyl cellulose and n-butyl glycolate have commercial significance.
n-Butyl Glycolate
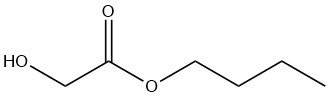
n-Butyl glycolate is a colorless liquid that is miscible with most organic solvents. Its solubility in water is limited to 8% by weight at 20 °C, although the compound itself can contain up to 25% by weight of water.
Production of n-butyl glycolate
The production of n-butyl glycolate involves the treatment of sodium chloroacetate with n-butyl alcohol at temperatures ranging from 125 to 160 °C, followed by vacuum distillation.
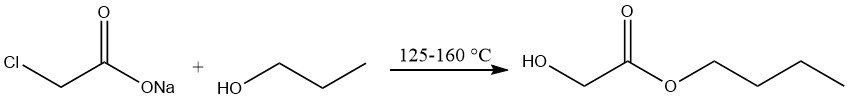
Uses of n-butyl glycolate
n-Butyl glycolate finds primary application as an additive in varnishes, valued for its low volatility. Some trade names for n-butyl glycolate include Polysolvan-O (Hoechst) and GB-Ester (Wacker).
It imparts smooth spreading properties and high gloss to nitrocellulose varnishes. In the case of acetyl cellulose, it effectively acts as a blush inhibitor under high humidity conditions.
Due to its favorable blending properties, n-butyl glycolate is also utilized as an additive in alkyd resins and oil-based paints.
n-Butyl glycolate is considered non-hazardous.
References
- Hydroxycarboxylic Acids, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_507
- Process for manufacture of glycolic acid. – https://patents.google.com/patent/US2152852A/en
- Glycolic Acid Production from Ethylene Glycol. – https://pubs.acs.org/doi/10.1021/acssuschemeng.1c03717


