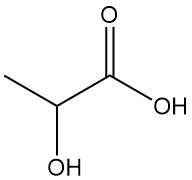
Lactic acid, also known as 2-hydroxypropionic acid, is a natural organic acid with the molecular formula CH3CH(OH)COOH. Pure lactic acid is a white, crystalline solid with a low melting point that is found in many biological systems, including the human body.
It plays an important role in cellular respiration.
Lactic acid was first isolated in 1780 by Swedish chemist Carl Wilhelm Scheele from sour milk. Initially, it appeared as an impure brownish syrup. Later, French chemist Michel Braconnot demonstrated that lactic acid could be produced by fermentation processes.
The year 1881 marked the establishment of industrial-scale lactic acid production in the USA.
Table of Contents
1. Physical Properties of Lactic Acid
Lactic acid [50-21-5] is the simplest hydroxycarboxylic acid containing an asymmetric carbon atom with a molar mass of 90.08 g/mol. It exists in three forms:
- Racemic mixture (DL): a non-optically active form containing equal amounts of the two optically active isomers.
- L-(+)-lactic acid (CAS number: 79-33-4): The optically active L-isomer.
- D-(-)-lactic acid (CAS number: 10326-41-7): The optically active D-isomer.
Pure, anhydrous racemic lactic acid is a white, crystalline solid with a low melting point, although the exact value is difficult to determine due to challenges in obtaining a completely anhydrous form. Reported values in the literature range from 18°C to 33°C. Lactic acid undergoes internal esterification to form lactoyllactic acid.
Isolation of individual optical isomers can be achieved by fractional crystallization of freshly distilled lactic acid. The melting point of pure optical isomers is around 52.7–52.8 °C. Anhydrous lactic acid has a boiling point of approximately 125–140 °C at 27 kPa.
Due to its hygroscopic property, lactic acid is typically obtained as a concentrated aqueous solution (up to 90 wt%) that is colorless, virtually odorless, and contains significant amounts of lactoyllactic acid and other lactic acid oligomers.
This solution is completely miscible with water, ethanol, diethyl ether, and other water-miscible organic solvents. It is practically insoluble in benzene and chloroform.
Historically, synthetic lactic acid has been a racemic mixture. However, lactic acid produced by fermentation is optically active, with the specific isomer produced depending on the type of bacteria involved.
Lactic acid has a dissociation constant (K) of 1.38 x 10-4 at 25°C, corresponding to a pKa of 3.86. A 10 wt% aqueous solution of lactic acid has a pH of approximately 1.75.
Density, viscosity, refractive index, and conductivity data for aqueous lactic acid solutions of varying concentrations are provided in Table 1.
| Concentration, wt% | Density, g/mL | Viscosity, mPa·s | Refractive index | Conductivity, mS/cm |
|---|---|---|---|---|
| 6.29 | 1.0115 | 1.042 | 1.3390 | 3.670 |
| 25.02 | 1.0570 | 1.725 | 1.3586 | 3.823 |
| 54.94 | 1.1302 | 4.68 | 1.3909 | 1.530 |
| 88.60 | 1.2006 | 36.9 | 1.4244 | 0.0567 |
2. Chemical Reactions of Lactic Acid
2.1. Internal Esterification and Polymerization
Lactic acid, possessing both hydroxyl and carboxylic acid functional groups, undergoes internal esterification to form lactoyllactic acid (2-(2-hydroxypropanoyloxy)propanoic acid) (1) or lactide (2), a cyclic ester, by bimolecular esterification.
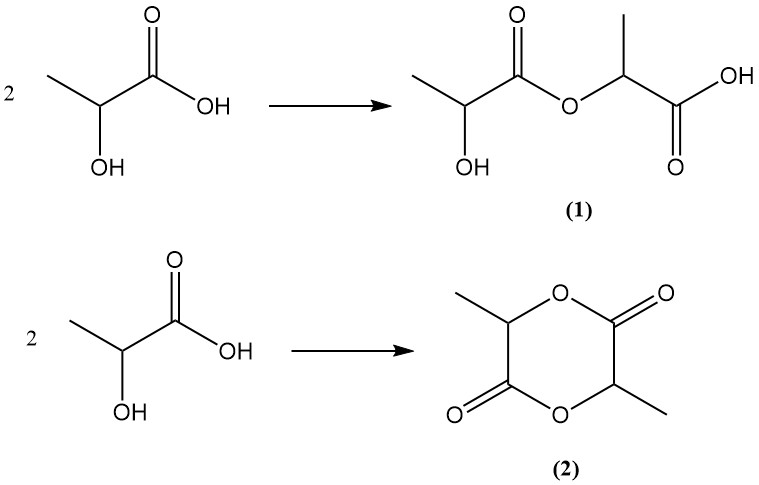
Lactide exists in three stereoisomeric forms: L,L-lactide, D,D-lactide, and D,L-lactide, depending on the chirality of the constituent lactic acid units.
Polylactic acids can be produced by further condensation of lactoyllactic acid. These are linear polyesters with varying chain lengths. , identical chemical structures can be obtained via lactide’s ring-opening polymerization, in which case the term “polylactide” is typically used.
Commercially available aqueous lactic acid solutions contain varying proportions of lactoyllactic acid and polylactic acids, influenced by concentration and storage time.
Dilute solutions (around 6.5 wt%) may contain approximately 0.2 wt% lactoyllactic acid, while concentrated solutions (88 wt%) might have less than 60% free lactic acid, and even 100% lactic acid solutions may only contain 32% of the free monomeric form.
2.2. Esterification with Alcohols
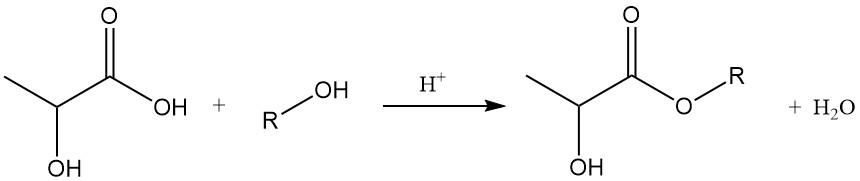
Lactates of lower alcohols can be readily synthesized by direct reaction with the corresponding alcohol. The reaction yield can be improved by removing the generated water through azeotropic distillation. Alternatively, lactate esters can be produced by minimal water-generating reactions between alcohols and polylactic acid.
2.3. Other Reactions
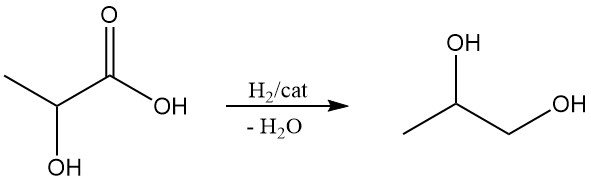
Lactic acid has the potential to be a cost-effective starting material for propylene glycol (1,2-propanediol) and acrylic acid, two important chemicals. Propylene glycol can be obtained by the reduction of lactic acid, and the dehydration of lactic acid produces acrylic acid.
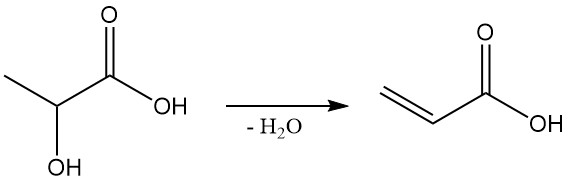
Additionally, lactic acid plays a significant role in biological processes.
3. Production of Lactic Acid
Lactic acid is produced commercially by either fermentation or synthetic methods. However, since approximately 1995, the fermentation process has dominated new production capacity. This shift is driven by the ability of fermentation to generate lactic acid with high chiral purity, a critical factor for applications in the food and polymer industries.
3.1. Production of Lactic Acid by Fermentation
Lactic acid production by fermentation uses carbohydrates, nutrients, and specific microorganisms.
The carbohydrates used consist of primarily hexoses (glucose, fructose) or easily degradable hexose precursors (corn syrups, molasses, sugar beet juice, whey). Starches from rice, wheat, corn, and potato are also employed.
As the hydrolysis technology of lignocellulosic materials advances, these raw material alogside pentose sugars (xylose, arabinose) may become a low-cost source of carbohydrates.
Various microorganisms are capable of high-volume lactic acid production, including Lactobacillus, Bacillus, and Rhizopus strains. Rhizopus fungi offer the advantage of not requiring complex nitrogen sources but typically yield less lactic acid compared to bacterial strains.
Bacterial strains generally perform anaerobic fermentation, while Rhizopus uses aerobic processes. Homofermentative bacterial strains that minimize by-product formation are preferred.
Microorganisms used in the fermentation require soluble peptides, amino acids, phosphates, ammonium salts, and vitamins. Complex nitrogen sources like yeast extract, corn steep liquor, or peptones (soy, meat) are often used in small quantities for simpler lactic acid purification.
The broth pH needs to be maintained between 5.0 and 6.5 during fermentation. Neutralizing agents like calcium hydroxide, carbonate, or sodium/ammonium hydroxide are used to counteract lactic acid production, resulting in the formation of calcium lactate, ammonium lactate, or sodium lactate salts within the broth.
Industrial-scale production necessitates large quantities of bacterial inoculum cultivated in a dedicated seed train. Strict measures are required to minimize contamination in the seed train and the production vessels to ensure high yields and chiral purity.

Lactic acid yields typically range from 85% to 95% based on the fermentable sugars. Common fermentation byproducts like formic acid and acetic acid are usually present in concentrations below 0.5 wt%.
Recent advancements in fermentation technology involve genetically engineered yeast strains for lactic acid production under neutral or low-pH conditions. These engineered microorganisms offer benefits like defined media requirements and the ability to produce lactic acid directly (not lactate salts) within the broth.
Low-pH fermentation potentially eliminates costs associated with neutralizing agents, acidifying agents, and byproduct salt disposal.
3.2. Lactic Acid Purification
Following fermentation, the broth containing lactic acid requires purification for specific end uses. The extent of purification varies depending on the application. Food applications may require minimal purification, while applications like polylactide monomer production necessitate extensive purification.
The choice of nutrients and microorganisms during fermentation significantly impacts downstream purification steps. Complex nitrogen sources and byproducts from microorganisms contribute to impurities that need to be removed. Unit operations are used to eliminate residual proteins, amino acids, sugars, cations, anions, and other organic acids.
Removing microorganisms from the broth is essential in all cases. The removal technique depends on the specific microorganism used in fermentation.
- Bacterial strains can be removed through flocculation under alkaline conditions or ultrafiltration.
- For Rhizopus, careful control of cell morphology within the fermenter is crucial for efficient separation on filters.
Lactate salts formed during fermentation must be converted to lactic acid. A common, large-scale method involves adding sulfuric acid to calcium lactate. This method generates a low-value calcium sulfate (gypsum) byproduct with limited water solubility.
Considerable research has focused on alternative processes to avoid byproduct salt formation, such as water-splitting electrodialysis and carbon dioxide-assisted liquid-liquid extraction.
For certain food applications, the broth is passed through activated carbon and ion exchange resins for purification. Following this step, the broth is concentrated to meet customer specifications.
Pharmaceutical, polymer, and other applications require further purification of lactic acid. Several large-scale processes are employed, with liquid-liquid extraction using long-chain tertiary amines in the organic phase being one method of interest.
Here, lactic acid is selectively extracted into the organic phase at low temperature and then back-extracted into water at high temperature to yield a high-quality lactic acid stream. Acid-amine complexation contributes to high selectivity for lactic acid over residual sugars and proteins.
Lactic acid distillation is achievable under specific operating conditions that minimize the formation of lactic acid oligomers, which can limit yield. Distilling a lactate ester, such as ethyl lactate, can also produce high-quality lactic acid but introduces the additional complexity of esterification and hydrolysis reactions.
Significant research has explored the use of membranes for lactic acid purification, including electrodialysis, and anion exchange. These technologies may play a role in future lactic acid production facilities.
3.3. Synthesis of Lactic Acid
While fermentation has dominated lactic acid production since the mid-1990s, synthetic processes have also existed since the 1960s. The current industrial method for manufacturing lactic acid involves the reaction of acetaldehyde with hydrogen cyanide, followed by the hydrolysis of the resulting lactonitrile.
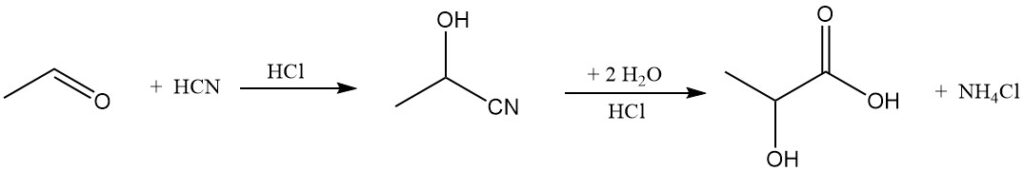
Musashino currently stands as the last major producer of synthetic lactic acid. However, the potential development of a cost-effective synthetic route that uses an enantioselective catalyst to generate chirally pure lactic acid could lead to a partial replacement of fermentation methods in the future.
4. Uses of Lactic Acid
Lactic acid and its derivatives (salts and esters) are used in three primary sectors: food, polymers, and industry.
4.1. Uses in Food
Traditionally, the food industry has been the largest consumer of lactic acid and lactate salts. Lactic acid is primarily used as an acidulant and preservative. Its mild acidic taste complements various foods without overpowering other flavors.
Additionally, being a naturally occurring substance, lactic acid doesn’t introduce foreign elements into food. Its highly soluble salts make them suitable partial replacements for acids in buffering systems.
Lactic acid and its salts are widely used in various food products, including beverages, candies, meat, and sauces. Calcium lactate can be added to foods to make calcium-enhanced products. Steroyl lactate and its sodium and calcium salts are used in bread.
4.2. Uses in Polymers
Lactic acid is a monomer used for the production of polylactic acid, or polylactide. NatureWorks LLC is a major producer of polylactic acid, which is synthesized via ring-opening polymerization of lactide. Lactide itself is formed by the condensation of two molecules of lactic acid.
Uses of polylactic acid include food and beverage containers, films and rigid containers for packaging, and serviceware like cups, plates, and utensils. Polylactic acid can be spun into fibers for use in clothing, fiberfill (pillows, comforters), carpets, and nonwoven applications such as wipes.
4.3. Industrial Uses
Lactic acid and its derivatives are used in metal plating, cosmetics, textiles, and the leather industry. Lactate esters are used in the production of paints and inks, electronics, and metal cleaning solutions. Lactic acid is also used in agriculture as animal feed.
5. Toxicology of Lactic Acid
Lactic acid is a nontoxic, naturally occurring edible acid that is used as an acidulant in the food industry. However, due to its acidic nature, it can cause irritation upon contact with the eyes or broken skin. In such cases, immediate flushing with water is recommended.
Oral toxicity is not a major concern under normal usage conditions. However, ingesting large quantities can be harmful, with an LD50 (lethal dose 50%) of 3730 mg/kg in rats. As a general safety precaution, avoid contact with the eyes and skin, and do not ingest large quantities.
References
- Lactic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a15_097.pub3
- Technological and economic potential of poly(lactic acid) and lactic acid derivatives


