Production Methods of Aliphatic Aldehydes
Aldehydes are denoted by the general chemical formula RCHO, where R may represent hydrogen or a diverse range of aliphatic, aromatic, or heterocyclic groups. In accordance with IUPAC nomenclature, aldehydes are recognized by the “al” suffix; but, many aldehydes are still called by their common names.
The primary method of producing aldehydes is oxo synthesis, achieved by mild oxidation (dehydrogenation) of primary alcohols and specialized olefin oxidation processes. In the essential oils of various plants, trace amounts of aldehydes occur naturally. Acetaldehyde, a byproduct of alcohol fermentation, forms by the decarboxylation of the intermediary pyruvic acid.
Aldehydes have important biological roles, such as 11-cis-retinal’s involvement in the visual process and pyridoxal’s participation in amino acid transamination.
The isolation of aldehydes from natural sources holds commercial significance in only a few cases, such as the production of longer-chain fragrance aldehydes.
Table of Contents
1. Production of Saturated Aldehydes
While numerous methods for synthesizing aldehydes have been discovered, only a select few are used at an industrial scale. This is often due to the availability of suitable raw materials. The following processes are the most significant for preparing saturated aliphatic aldehydes:
- Hydroformylation of olefins, commonly known as oxo synthesis.
- Dehydrogenation or oxidation of primary alcohols, primarily employed for the production of formaldehyde from methanol.
- Hydration of acetylene, leading to the formation of acetaldehyde (also known as ethanal).
- Oxidation of ethylene to yield acetaldehyde.
- Oxidation of saturated hydrocarbons, particularly C3 and C4 hydrocarbons, to produce lower aldehydes.
Specific syntheses for producing aldehydes used in the perfume industry are also important.
1.1. Oxo Synthesis
The oxo synthesis stands out as the most significant method for producing aldehydes that consist of at least three carbon atoms (referred to as Oxo Synthesis). In this particular process, olefins undergo a reaction with synthesis gas, which is a mixture of carbon monoxide (CO) and hydrogen (H2). As a result of this reaction, aldehydes with one additional carbon atom compared to the starting olefin are formed.

The formation of pure products occurs only when dealing with symmetrical or sterically hindered olefin molecules. However, in other cases, a mixture of straight-chain and branched compounds is obtained. By carefully selecting suitable catalysts and controlling the reaction conditions, the ratio between normal (n) and iso (branched) aldehyde products can be adjusted across a wide range.
1.2. Dehydrogenation/Oxidation of Primary Alcohols
Dehydrogenation, oxidation, and oxidative dehydrogenation reactions are represented by the following equations:
- RCH2OH → RCHO + H2; ΔH = +84 kJ/mol for R = CH3 (Equation 1)
- RCH2OH + 1/2 O2 → RCHO + H2O; ΔH = -159 kJ/mol for R = H (Equation 2)
- RCH2OH → RCHO + H2; H2 + 1/2 O2 → H2O; ΔH = -159 kJ/mol for R = H (Equation 3)
Dehydrogenation: The endothermic dehydrogenation reaction of alcohols occurs at atmospheric pressure and temperatures ranging from 250 to 400°C, typically utilizing Cu or Ag catalysts. These catalysts are often activated with elements like Zr, Co, or Cr.
The process offers the advantage of simultaneous hydrogen recovery, which can be used without additional purification. It is an equilibrium reaction, necessitating high temperatures and short residence times for economic efficiency.
The process finds commercial use in the preparation of acetaldehyde from ethanol. Gas-phase dehydrogenation with a copper catalyst activated by cerium is conducted at atmospheric pressure and 270-300°C, converting 25-50% of ethanol per throughput, with a 90-95% selectivity for acetaldehyde, along with the formation of byproducts such as ethyl acetate, ethylene, crotonaldehyde, and higher alcohols.
Oxidation: The oxidation process described by Equation (2) is carried out using an excess of air or oxygen and a catalyst containing 18-19 wt% Fe2O3 and 81-82 wt% MoO3 at temperatures ranging from 350 to 450°C. This method is employed in the production of formaldehyde.
Oxidative Dehydrogenation: Equation (3) combines the endothermic dehydrogenation of the alcohol with the exothermic combustion of the hydrogen formed, resulting in an overall exothermic reaction.
In the industrial process, both reactions occur simultaneously when substoichiometric quantities of oxygen or air are used. It is crucial to consider the explosion ranges of alcohol-air mixtures during oxidation and oxidative dehydrogenation.
Oxidative dehydrogenation is the most important process for producing aldehydes from alcohols. Silver catalysts are preferred, but copper catalysts are also used. In formaldehyde production from methanol, silver crystals (grain size 0.2-3 mm), silver nets, or silver on Al2O3 achieve 75-99% conversion at temperatures of 500-720°C and residence times less than 0.01 s.
In 2006, about 7% of Western Europe’s acetaldehyde production was from ethanol. Silver and copper were the primary catalysts used, with ethanol conversions of 30-50 % per throughput and acetaldehyde selectivity of 85-95 %. The reaction temperature ranged between 300 and 600 °C depending on the amount of air. Byproducts included ethyl acetate, formic acid, acetic acid, and carbon dioxide.
Fragrance Aldehydes: Dehydrogenation and oxidation processes are also preferred for synthesizing fragrance aldehydes. A specialized process allows for the catalytic dehydrogenation of C5-C14 alcohols in the presence of hydrogen and air, using copper or silver catalysts, possibly combined with Zn, Cr, or Cr2O3.
Other catalyst systems described in the literature include Cu/MgO, Ag/Na2O on supports, mixtures of MnO, NiO on MgO, or CuCl with a nitrogen-containing ligand such as 2,20-bipyridyl.
1.3. Oxidation of Hydrocarbons
Celanese has developed a process for the oxidation of C3 and C4 alkanes, but this method produces a complex reaction mixture that requires expensive extraction and distillation steps.
In this process, propane and propane-butane mixtures are reacted in the gas phase at temperatures ranging from 425 to 460°C and pressures of 0.7 to 0.8 MPa. The conversion rate is approximately 20%, with an oxygen deficiency present during the reaction. The reaction proceeds through a radical mechanism.
The reaction mixture obtained from this process primarily consists of acetaldehyde (≈ 20%), formaldehyde (≈ 15%), methanol (≈ 19%), and organic acids (≈11%).
However, despite these components, the process is considered technologically outdated. Moreover, the oxidation of methane or ethane using this method does not hold any practical significance.
1.4. Oxidation of Olefins
The primary and crucial industrial method for producing acetaldehyde is the partial oxidation of ethylene in the aqueous phase, carried out in the presence of palladium and copper chlorides. This process is known as the Wacker-Hoechst process.
Another oxidation process used to prepare aldehydes from olefins is the acrolein synthesis, which involves the conversion of propene.
1.5. Miscellaneous Processes
The process that involves the addition of water to acetylene to produce acetaldehyde has lost its significance in comparison to alternative processes based on ethylene or ethanol. In Western Europe, the last plants using this process were closed in 1980.
The diminished importance of the acetylene-based process is attributed to the ready availability and lower cost of ethylene, as well as the superior selectivity of its conversion. Additionally, the use of environmentally harmful mercury sulfate as a catalyst has contributed to the process becoming practically obsolete.
2. Production of Unsaturated Aldehydes
Lower α,β-unsaturated aldehydes, including acrolein, crotonaldehyde, or 2-ethyl-2-hexenal, are primarily obtained by synthetic processes. However, certain essential oils can serve as raw materials for higher homologues, such as citral or citronellal.
There are two main industrial processes for manufacturing α,β-unsaturated aldehydes:
1. Oxidation of olefins: This process is used to prepare acrolein.
2. Dehydration of aldols obtained by aldol condensation of saturated aldehydes: This method is employed to produce crotonaldehyde and 2-ethyl-2-hexenal.
In addition to these processes, there are specific syntheses used to prepare aldehydes for the perfume industry, such as:
1. Dehydrogenation of unsaturated alcohols: This process is utilized in the preparation of citral from geraniol.
2. Reduction of unsaturated acids: This method is used to produce undecylenealdehyde from undecylenic acid.
2.1. Oxidation of Olefins
The direct oxidation of olefins, specifically the oxidation of propene, is of great industrial significance for the production of acrolein. This oxidation reaction occurs at temperatures ranging from 300 to 480°C and employs variously modified Bi-Mo oxide catalysts. The feedstock used is a gaseous mixture containing propene, air, and water vapor in a molar ratio of approximately 1:10:2.
During this process, conversion rates of up to 98% can be achieved, with acrolein yields ranging from 78% to 92%. However, alongside acrolein, some byproducts are formed, including acetaldehyde, acetic acid, and acrylic acid.
Commercially available acrolein typically has a purity level of 95% to 97%. To prevent unwanted reactions and maintain stability throughout the processing steps, a stabilizer is typically added. Hydroquinone is commonly used as the stabilizer in these processes.
2.2. Dehydration of Aldols
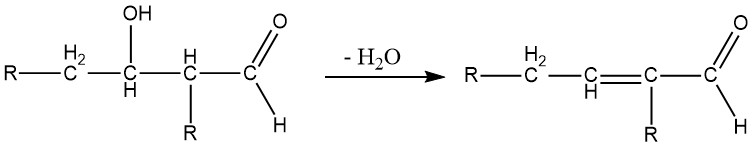
The β-hydroxyaldehydes (aldols) are intermediate compounds formed in the aldol reaction and are highly unstable. They readily decompose, losing water, to form α,β-unsaturated aldehydes.
Depending on the reaction conditions, the aldol reaction can directly lead to the formation of unsaturated compounds. This process is commonly used for synthesizing crotonaldehyde from acetaldehyde, 2-methyl-2-pentenal from propionaldehyde, and 2-ethyl-2-hexenal from butanal.
The aldol condensation of acetaldehyde with catalytic amounts of dilute sodium hydroxide is typically conducted at 20-25°C. The reaction is quenched with acetic acid to stop further reactions. In the subsequent distillation step, water is removed from the acetaldol, resulting in crotonaldehyde as the main product. The selectivity towards crotonaldehyde can reach values of up to 95%.

The aldol reaction can involve two identical aldehyde molecules or two different aldehyde species. In the latter case, a mixture of products is often obtained. However, by carefully selecting the reactants and reaction conditions, it is possible to obtain the desired compound as the main product.
For example, acrolein was previously prepared by the reaction of formaldehyde with acetaldehyde.

Similarly, higher 2-methylenealkanals (2-alkylacroleins) can be obtained by reacting formaldehyde with longer-chain aldehydes.
2.3. Miscellaneous Processes
For the preparation of certain unsaturated aroma aldehydes, the corresponding alcohols undergo selective dehydrogenation using copper, copper-zinc, or noble metal catalysts. These processes are preferably conducted under reduced pressure and are commonly used in the production of citral, citronellal, and hydroxycitronellal.
Another industrial method of interest for synthesizing unsaturated aldehydes is the Claissen rearrangement of allyl vinyl ethers. Allyl vinyl ethers are formed as intermediates in this process according to the following reaction scheme:

2-Alkenals can also be obtained by reacting unsaturated alkyl halides with the sodium salts of secondary nitrohydrocarbons. For instance, citral can be obtained in an 80% yield from 1-halo-3,7-dimethylocta-2,6-diene.
An additional approach involves treating acetals with vinyl ethers in the presence of boron trifluoride. This results in the formation of corresponding β-alkoxyacetals. Subsequent treatment with acids leads to the conversion of β-alkoxyacetals into α,β-unsaturated aldehydes.
3. Production of Hydroxyaldehydes
3.1. Aldol Condensation
The aldol condensation, first reported in 1872 by A. Wurtz, can be described in the case of base-catalyzed reactions by the following mechanism:
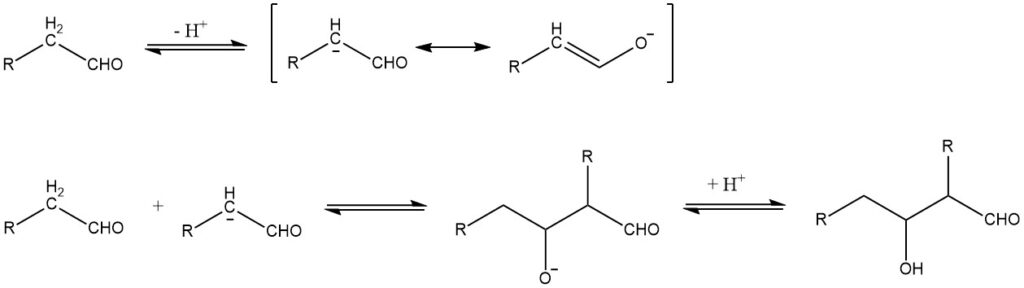
The aldol condensation is a reversible reaction and can be catalyzed not only by bases but also by acids. It is possible only for aldehydes with at least one α-hydrogen atom. Aldols obtained from aldehydes with more than one α-hydrogen atom are usually unstable and lose water to form α,β-unsaturated aldehydes:
When two different aldehydes with α-hydrogen atoms undergo aldol addition, all four possible aldol species are generally formed in varying amounts.
For industrial purposes, reactions in which only one reactant possesses α-hydrogen atoms are more significant. In such cases, the second reactant is often formaldehyde. Reactions to prepare mono- or polymethylolalkanals from formaldehyde and alkanals are common in industrial processes.
These exothermic reactions are typically conducted in the liquid phase, and catalysts like aqueous solutions of sodium hydroxide or alkali carbonates are frequently employed.
Various other catalyst systems have also been described, including zinc- or magnesium-containing zeolites, alkali hydroxides combined with phase-transfer catalysts, tertiary amines, and basic ion exchange resins.
Typical byproducts of aldol condensation, aside from the dimeric aldols, aldoxanes, and α,β-unsaturated aldehydes mentioned earlier, include cyclic acetals, Tishchenko esters, etc. Reactions with formaldehyde can lead to additional complications, as a reduction of the aldol by a crossed Cannizzaro reaction might occur.
G. Wittig discovered a modified version of the classic aldol reaction involving the treatment of carbonyl compounds with metalated imines. In this case, α,β-unsaturated aldehydes are predominantly formed, and this method has shown its value, particularly in the field of natural products.
3.2. Miscellaneous Processes
The addition of water to α,β-unsaturated aldehydes results in the formation of β-hydroxyaldehydes. This reaction is of industrial importance and is used for the synthesis of 1,3-propanediol.
For instance, 3-hydroxypropanal can also be obtained by the hydroformylation of ethylene oxide, while 4-hydroxybutanal is obtained through the hydroformylation of allyl alcohol. The hydroformylation of unsaturated alcohols to produce hydroxyaldehydes has been thoroughly reviewed.
Additionally, when 2,3-dihydro-1,4-pyran is hydrolyzed, it yields 5-hydroxypentanal, which exists in equilibrium with its cyclic hemiacetal form. For example, 2-phenyl-2,3-dihydropyran, produced from acrolein and styrene by a Diels-Alder synthesis, can be cleaved using dilute sulfuric acid to produce 5-phenyl-5-hydroxypentanal.
4. Production of Dialdehydes
Numerous syntheses of dialdehydes are documented in patent literature, but only a limited number of them hold commercial significance, largely owing to their lack of selectivity. The key processes utilized for the production of saturated aliphatic and cycloaliphatic dialdehydes are as follows:
- Oxidation of ethylene glycol
- Oxidation of acetaldehyde
- Hydroformylation of dienes
- Oxidative ring opening of cycloalkenes
- Addition of methyl vinyl ether to acrolein (resulting in glutardialdehyde)
4.1. Hydroformylation of dienes
Hydroformylation of dienes leads to diverse products, influenced by both the diene’s structure and the catalyst employed. When dealing with conjugated dienes like 1,3-butadiene using unmodified cobalt or rhodium catalysts, saturated monoaldehydes or monoalcohols are produced.
Initially, the single addition of CO/H2 generates the unsaturated monoaldehyde. Subsequently, hydroformylation of the unsaturated aldehyde competes with hydrogenation and isomerization of the double bond.
On the other hand, nonconjugated dienes typically isomerize to the thermodynamically more stable α,β-unsaturated aldehydes after the single addition of CO/H2. Consequently, nonconjugated dienes with widely separated (> C6), preferably terminal double bonds, are particularly useful for the synthesis of dialdehydes via hydroformylation.
4.2. Oxidative Ring Opening of Cycloalkenes
Cycloalkenes, when exposed to catalysts like tungstic acid, undergo a reaction with hydrogen peroxide, leading to the formation of the corresponding epoxides and resulting in linear α,ω-dialdehydes.

In certain cases, the addition of boron compounds facilitates the reaction.
4.3. Miscellaneous Processes
Similar to the process of forming monoaldehydes through dehydrogenation, the dehydrogenation of diols results in the production of dialdehydes.
There are other pathways as well, including the ozonization of cycloalkenes and the dimerization of unsaturated aldehydes. Additionally, hydroformylation of unsaturated acetals leads to the corresponding dialdehydes.
5. Production of Acetals
The primary method employed for the preparation of acetals is the reaction of an aldehyde with an alcohol. Typically, an unstable hemiacetal is formed as an intermediate product due to the equilibrium nature of the reaction.
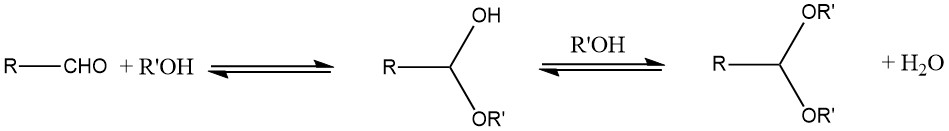
To enhance the yield, the water produced during the reaction must be removed using azeotropic distillation or water-adsorbing agents, like molecular sieves. In general, cyclic acetals tend to offer better yields than open-chain acetals.
Anhydrous sulfuric acid or p-toluene sulfonic acid serve as common catalysts, although other options such as inorganic acids, oxalic or adipic acid, ion-exchange resins, or molecular sieves can be used as well.
During the acetalization of saturated aliphatic aldehydes containing an α-hydrogen atom, the formation of 1-alkenyl ethers can occur if the reaction temperature is too high.
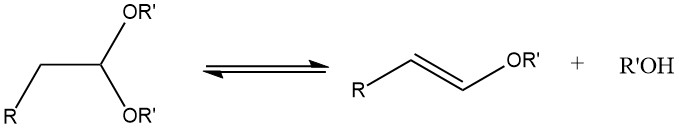
Acetals can be synthesized with good yields from most aldehydes by treating them with orthoformates, mainly the methyl and ethyl esters, using strong acids as catalysts, as per the process developed by L. Claisen.
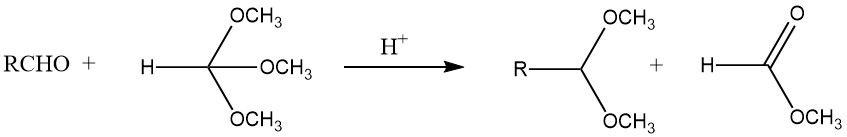
Furthermore, acetals can be obtained by the reaction of acetylene with alcohols, the addition of alcohol to vinyl ethers, and the treatment of geminal dihalides with alkoxides. Aldehydes and oxiranes yield 1,3-dioxolanes, while pyrocatechols and dichloromethane form 1,3-benzodioxoles.
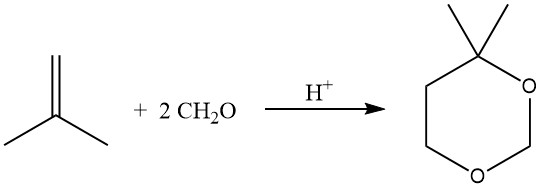
The acid-catalyzed reaction of olefins with formaldehyde results in the formation of 1,3-dioxanes (Prins reaction), carried out at 55–75°C with an excess of formaldehyde.
Under hydroformylation conditions, the reaction of olefins with alcohols, often using phase-transfer catalysts, can also yield acetals.
Acetaldehyde dimethyl acetal is obtained at 200°C from methanol and synthesis gas in the presence of catalyst, with approximately 60% methanol conversion and 80–85% selectivity. Main byproducts include acetaldehyde and ethyl acetate.
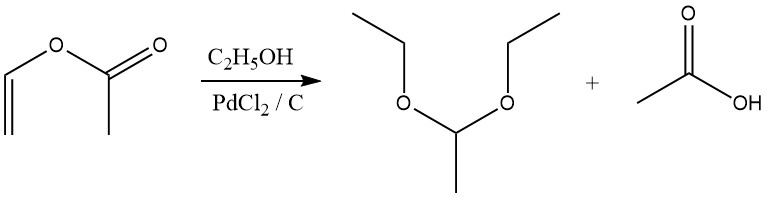
Acetals are also formed by reaction of Vinyl esters with alcohols in the presence of a palladium chloride catalyst.
References
- Aldehydes, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_321.pub3
- Production of aliphatic aldehydes. – https://patents.google.com/patent/US2623905A/en
