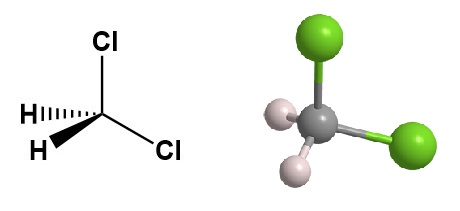
Dichloromethane, also known as methylene chloride, or DCM, is a colorless, highly volatile liquid with a sweet, chloroform-like odor. Its chemical formula is CH2Cl2, and it is a widely used industrial solvent with a variety of applications. It represents 25% of the total production of chloromethanes (CH3Cl, CH2Cl2, CHCl3, and CCl4).
Table of Contents
1. Physical Properties of Dichloromethane
Dichloromethane is a colorless, volatile liquid with a slightly sweet smell that can form azeotropic mixtures with a number of substances.
Dichloromethane (DCM), despite possessing flammability limits in air, exhibits unique behavior that challenges conventional classification. While its vapor flammability range exists between 13-22% at 20°C, ignition is difficult due to the high ignition energy requirement (9100 mJ for an 18% mixture).
This contrasts with typical flammable solvents, rendering DCM resistant to ignition by low-energy sources like cigarettes or sparks. However, high-energy sources like torches or welding flames pose a fire risk.
It lacks a measurable flash point according to established testing standards, further complicating its flammability profile. Additionally, it can elevate the flash points of other flammable liquids when mixed, offering potential fire safety benefits in specific cases.
Given these complexities, dichloromethane falls within temperature class 1 (ATEX) due to its flammability limits.
Its principal physical properties are listed in the following table:
| Property | Value |
|---|---|
| Molecular weight (g/mol) | 84.93 |
| Boiling point at 1 bar (°C) | 40.0 |
| Melting point (°C) | −95.1 |
| Vapor pressure at 20 °C (mbar) | 467 |
| Enthalpy of vaporization (kJ/mol) | 27.99 |
| Enthalpy of fusion at melting point (kJ/mol) | 6.2 |
| Density of liquid at 20 °C (kg/m³) | 1326.6 |
| Density of vapor at boiling point (kg/m³) | 3.406 |
| Cubic expansion coefficient of liquid (0–40°C) (K⁻¹) | 0.00137 |
| Enthalpy of formation of vapor at 25°C, 1 bar (kJ/mol) | −92.47 |
| Gibbs free energy of formation of vapor at 25°C, 1 bar (kJ/mol) | −65.87 |
| Specific heat capacity of vapor at 25°C, 1 bar (kJ kg⁻¹ K⁻¹) | 0.600 |
| Enthalpy of formation of liquid at 25°C (kJ/mol) | −121.46 |
| Gibbs free energy of formation of liquid at 25°C (kJ/mol) | −67.26 |
| Specific heat capacity of liquid at 25 °C (kJ kg⁻¹ K⁻¹) | 1.177 |
| Critical temperature (Tc) (°C) | 245 |
| Critical pressure (atm) | 60.9 |
| Critical volume (mL/mol) | 0.1815 |
| Critical compressibility factor | 0.2731 |
| Thermal conductivity of vapor (W K⁻¹ m⁻¹) | 0.00758 |
| Thermal conductivity of liquid at 20°C (W K⁻¹ m⁻¹) | 0.156 |
| Surface tension at 20°C (10⁻³ N/m) | 28.2 |
| Viscosity of liquid at 20°C (cP) | 0.425 |
| Dipole moment | 1.59 |
| Refractive index of liquid at 25°C | 1.4244 |
| Dielectric constant of vapor at 20°C | 1.01 |
| Dielectric constant of liquid at 20°C | 9.10 |
| Ignition temperature (°C) | 605 |
| Limits of ignition in air, lower vol% | 13 |
| Limits of ignition in air, upper vol% | 22 |
| Partition coefficient air/water at 20°C | 0.12 |
| Partition coefficient n-octanol/water at 20°C as log Pow | 1.25 |
2. Chemical Properties of Dichloromethane
Dichloromethane is thermally stable, tolerating short-term exposure to temperatures exceeding 140 °C and even 120 °C in the presence of oxygen. However, its decomposition depends on various other factors:
- Exposure time: Prolonged heating accelerates decomposition.
- Moisture and other chemicals: The presence of moisture, rust, or specific chemicals can promote decomposition.
- Container material: Mild steel, stainless steel, and glass offer better compatibility than materials like aluminum.
In the presence of heat or flames, dichloromethane decomposes to hydrogen chloride and traces of phosgene (with oxygen). Photooxidation of DCM leads to carbon dioxide, hydrogen chloride, and small amounts of phosgene. Reactions with nitrogen dioxide produce carbon monoxide, nitrogen monoxide, and hydrogen chloride.
While stable with most construction metals, dichloromethane reacts with zinc, aluminum, magnesium, and their alloys in Grignard-like reactions, causing corrosion. Stabilized dichloromethane is recommended for contact with such metals.
Dichloromethane undergoes negligible hydrolysis during evaporation but slowly hydrolyzes over time at ambient temperatures, generating formaldehyde and hydrogen chloride. This explains the observed rusting on steel surfaces.
Suitable stabilizers, acting as acid and radical scavengers, are crucial for storage and use. Steam treatment at elevated pressure readily hydrolyzes dichloromethane.
Thermal or photochemical chlorination processes can introduce more chlorine atoms to dichloromethane, which is used for the production of more substituted methanes like chloroform and tetrachloromethane.
Bromine or hydrogen bromide, with aluminum chloride as catalysts, can replace chlorine by bromine forming chlorobromomethane or dibromomethane.
Reaction of DCM with hydrogen fluoride yields difluoromethane (HFC-32).
Using aluminum as a catalyst at 220 °C and 90 MPa, the carbonylation of dichloromethane produces chloroacetyl chloride.
An alcoholic ammonia solution produces hexamethylenetetramine with DCM at 125 °C.
Reactions with phenolates mimic formaldehyde-phenol reactions.
3. Production of Dichloromethane
Dichloromethane is produced industrially by the direct chlorination of methane and monochloromethane with chlorine. This process is conducted at high temperatures (400–500 °C) and generates a mixture of chlorinated methane derivatives, including chloromethane, chloroform, carbon tetrachloride, and DCM.
Dichloromethane is separated from the mixture and purified by distillation.
A detailed industrial process description can be found in the articles about chloroform and tetrachlorometane.

a) Loop reactor; b) Process gas cooler; c) Quench; d) Gas/liquid separator; e) HCl absorption; f) Neutralization system; g) Sulfuric acid drying column; h) Compressor; i) First condensation step; j) Second condenser; k) Condensate buffer vessel; l1–l4) Distillation columns for CH3Cl, CH2Cl2, CHCl3 and CCl4
Photochlorination emerges as a potential alternative for dichloromethane production. It uses a radical pathway to selectively convert monochloromethane to DCM at -20 °C using UV lamps, resulting in a product with minimal (2–3%) trichloromethane content.
This process cannot be used for the direct chlorination of methane. Studies report its effectiveness in purifying dichloromethane from C2 component impurities.
4. Uses of Dichloromethane
Dichloromethane is used in industrial applications, primarily as a solvent for the chemical synthesis, extraction, and purification of pharmaceutically active ingredients like antibiotics, vitamins, caffeine, and flavors.
It is employed in the manufacture of polycarbonate plastics, offering glass-like properties.
DCM acts as a co-foam-blowing agent in the production of soft polyurethane foams.
Dichloromethane’s ability to dissolve various organic compounds makes it valuable as a solvent for metal cleaning machines, for special adhesives and cleansers, and in laboratories.
DCM is used as a starting material for producing difluoromethane (HFC-32), a low-temperature refrigerant used in blends like R-407C and R-410A.
It has been used in paint strippers but has been restricted or banned in many industrialized nations due to safety concerns. Its high volatility and open-air applications in paint stripping can lead to uncontrolled exposure, posing health risks.
Safe use of DCM-based products is best ensured by trained professionals or in closed systems, as permitted in certain regions.
5. Toxicology of Dichloromethane
While dichloromethane (DCM) poses moderate toxicity upon ingestion, its primary danger lies in its effects on the eyes and skin. It can cause significant pain, but absorption is typically limited due to its rapid evaporation. Inhalation, however, presents the major exposure route.
High-Level Inhalation Effects:
- Exposure exceeding 1000 ppm leads to anesthesia and incoordination.
- Metabolism of DCM to carbon monoxide results in carboxyhemoglobin (COHb) formation, mimicking carbon monoxide poisoning.
- At acceptable exposure levels, the potential adverse effects of COHb likely impact only individuals with pre-existing cardiovascular or respiratory issues.
Other Toxicological Concerns:
- DCM exhibits limited teratogenicity (birth defects) in animals and weak mutagenic activity in vitro tests.
- In vivo genotoxicity (genetic damage) was not observed.
- While animal studies link inhalation exposure to lung and liver tumors in mice (not rats or hamsters), this carcinogenicity seems irrelevant to humans due to differing metabolic pathways.
- Recent research suggests hypoxia and altered circadian signaling as potential key factors in dichloromethane-induced mouse tumors.
- Despite the IARC classifying DCM as “probably carcinogenic to humans” based on theoretical metabolic similarities, available epidemiological data from occupational exposures up to 100 ppm do not show carcinogenic effects in humans.
- Occupational Exposure Limits (OELs) for dichloromethane typically range between 25 and 100 ppm, reflecting the need for careful handling and appropriate ventilation.
Reference
- Chloromethanes; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_233.pub4