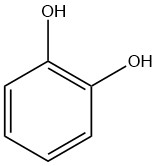
Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula C6H4(OH)2. It is a crystalline substance with a distinctive phenolic fragrance.
Catechol is an aromatic diol that was originally discovered in 1839 through the dry distillation of catechin by Reinsch. Today, it is synthesized industrially from phenol.
Catechol has been found in a range of sources, including raw beet sugar, crude wood tar, select species of eucalyptus, onions, and coal.
Catechol and its derivatives can be produced through a variety of techniques, such as dry distillation and other procedures, from substances such as tannin, lignin, wood, and bituminous coal. Additionally, it can be found in tobacco smoke and exists in the form of sulfuric ester in the urine of humans and horses.
Table of Contents
1. Production of Catechol
In the past, Catechol was primarily produced through the process of low-temperature carbonization of coal. However, this technique is presently used only on rare cases.
1.1. Production of Catechol by Hydrolysis of 2-Chlorophenol

In industrial settings, the production of catechol can be accomplished via the hydrolysis of 2-chlorophenol using a solution of barium hydroxide and sodium hydroxide. The recovery of barium, which is transformed into hydroxide from carbonate and subsequently recycled into the process, contributes to the complexity of the method. In response, alternative techniques that employ solely caustic alkali have been devised.
For instance, the reaction of 1 mole of 2-chlorophenol with 2.3 moles of a sodium hydroxide solution that ranges from 4-8% is carried out at 190°C for a duration of 3 hours within a copper autoclave, in the presence of copper(II) sulfate or copper(I) oxide. The selectivity for catechol is 81–86%, while the conversion of 2-chlorophenol ranges from 96–99%.
The resulting mixture is then neutralized using sulfuric acid, and the crude catechol formed is subsequently extracted. The solvent is recovered, and catechol is purified through distillation. This method of production was employed for industrial catechol production until 1973.
1.2. Production of Catechol by Hydroxylation of Phenol
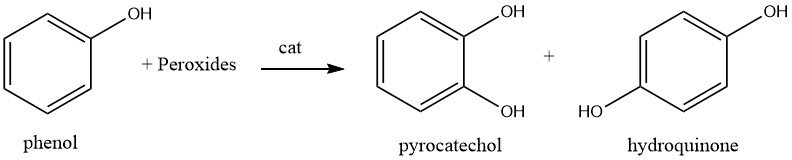
The production of catechol is currently carried out by the direct hydroxylation of phenol with peroxides, which also produces hydroquinone.
Three plants employing this method are currently operational worldwide, with each plant utilizing a distinct peroxide or catalyst.
Due to the exothermic nature of the reaction and the tendency of benzenediols to be more readily oxidized than phenol, the reaction is performed with a significant excess of phenol.
A plant in France, operated by Rhone-Poulenc, reacts phenol with 70% hydrogen peroxide (molar ratio 20:1) in the presence of phosphoric acid and catalytic amounts of perchloric acid at 90°C. This results in the production of catechol and hydroquinone in a ratio of approximately 3:2.
The reaction proceeds electrophilically, and phosphoric acid is employed as a masking reagent to prevent side reactions caused by trace amounts of metallic ions, such as the formation of resorcinol by a radical reaction that results in a lower yield.
Following the reaction, phosphoric and perchloric acids are removed by washing with water, and the reaction mixture is simultaneously extracted by diisopropyl ether, distilled, and continuously separated.
Brichima SpA (now Enichem) in Italy employs heavy metal compounds, such as small quantities of ferrocene and/or cobalt salts, as catalyst and reacts phenol with 60% aqueous hydrogen peroxide at 40°C. Catechol and hydroquinone are produced in the ratio of 1.5–4.1 through a free-radical chain mechanism that proceeds rapidly.
In Japan, Ube Industries produces catechol with hydroquinone by hydroxylation of phenol with ketone peroxide (methyl ethyl ketone peroxide) formed in situ from a ketone and hydrogen peroxide in the presence of an acid catalyst.
The process is carried out by adding a trace amount of acid, such as sulfuric or sulfonic acid, a small volume of ketone, and 60% aqueous hydrogen peroxide to phenol at 70°C. The ketone peroxide that is formed in situ reacts rapidly and electrophilically with phenol, and catechol and hydroquinone are produced in a molar ratio of approximately 3:2 in over 90% yield (based on phenol reacted).
When a solid acid such as clay is used as a catalyst, the molar ratio of catechol and hydroquinone is approximately 1:1. The use of a small amount of catalyst prevents corrosion, and the reaction mixture can be distilled without removing the catalyst after the reaction. The added ketone can be recycled to the process by recovering it through distillation.
The separation and purification methods employed in all three processes are fundamentally the same. The reaction mixture is separated through distillation in various distillation columns.
Water is removed, low-boiling fractions (solvents, ketone, etc.), and unreacted phenol are recovered and recycled, the catechol fraction is made into a flaked product, and hydroquinone is purified by recrystallization of the corresponding fraction from water.
1.3. Production of Catechol by Dehydrogenation of 1,2-Cyclohexanediol

Catechol can be synthesized with a 90% yield by dehydrogenation of 1,2-cyclohexanediol using a Pd/Te catalyst system at a temperature of 300 °C. It has been reported that catechol is produced as the sole product of this process.
Apart from this, catechol can be synthesized by employing the following methods:
- alkali fusion of 2-phenolsulfonic acid and phenol-2,4-disulfonic acid
- oxidation of salicylaldehyde with hydrogen peroxide in an aqueous alkaline solution
- demethylation of guaiacol using hydrobromic acid or aluminum chloride
- hydrolysis of 2-aminophenol using a hydrogen halide.
2. Chemical reactions of catechol
The dehydrogenation of 1,2-cyclohexanediol using a Pd/Te catalyst system at 300°C produces catechol as the sole product in a 90% yield.
Catechol exhibits distinct green coloration with iron-(III) chloride, and the addition of sodium hydroxide or ammonia results in a color change to red. Such characteristics are unique to catechol and can aid in its identification and detection.
Catechol forms stable coordination compounds with almost all metals, thereby serving as a useful analytical reagent for metal detection.
Alkali hydroxides, or carbonates, react with catechol, a weak acid, to produce mono- and disalts. Catechol’s heavy metal salts, particularly its lead salts, are insoluble in water. As a result, the reaction of catechol with lead acetate can facilitate the separation and quantitative analysis of catechol from its isomers hydroquinone and resorcinol.
As the strongest reducing agent among the three benzenediol isomers, catechol reacts with heavy metal salt solutions to produce fine elemental metal precipitates.
The oxidative cleavage of catechol using oxygen in the presence of copper(I) chloride and methanol results in the formation of cis,cis-Muconic acid monomethyl ester. Similarly, careful oxidation of catechol with silver oxide or silver carbonate on celite leads to the formation of 1,2-benzoquinone.
Catechol reacts with acyl halides to generate the corresponding mono- and diesters. These compounds are converted into phenolic ketones through Fries rearrangement, catalyzed by aluminum chloride.
The Reimer-Tiemann reaction with chloroform and alkali or the addition of glyoxylic acid and subsequent oxidative decarboxylation can introduce an aldehyde group into the aromatic nucleus .
The usual methods can be used to prepare mono- and diethers of catechol, which can undergo cyclization reactions due to their adjacent hydroxyl groups.
Methylenedioxybenzene and dibenzo-18-crown-6-polyether can be obtained by catechol’s reaction with dichloromethane and bis(2-chloroethyl) ether, respectively.
Catechol couples with aryldiazonium salts to form azo compounds that can be reduced to 4-aminocatechol.
Catechol can undergo condensation reactions with formaldehyde to produce bis(dihydroxyphenyl)methane and with phthalic anhydride to form polycyclic compounds such as hystazarin and alizarin.
Catechol can be alkylated, halogenated, nitrated, carboxylated, and sulfonated at the 3- and 4- positions.
3. Uses of catechol
Catechol is used as a photographic developer, an analytical reagent, and antioxidant. However, most of the catechol is used in the form of its derivatives. For example, O-methylation of catechol produces guaiacol (2-methoxyphenol) and veratrole (1,2-dimethoxybenzene).
Guaiacol is utilized to synthesize vanillin (4-hydroxy-3-methoxybenzaldehyde), which is used as a flavoring agent. Ethylvanillin (3-ethoxy-4-hydroxybenzaldehyde), derived from guethol (2-ethoxyphenol, a homologue of guaiacol), is a valuable aroma compound, as its flavor is 3–4 times stronger than that of vanillin.
Eugenol (2-methoxy-4-allylphenol), safrole (5-allyl-1,3-benzodioxole), and piperonal [3,4-(methylenedioxy)benzaldehyde], which can be prepared from the latter, serve as useful fragrances in perfumery.
Potassium guaiacol sulfonate (4-hydroxy-3-methoxy-benzenesulfonic acid monopotassium salt) and guaiacol glyceryl ether [3-(2-methoxyphenoxy)-1,2-propanediol] are used as expectorants in medicine.
L-α-Methyldopa (3-hydroxy-α-methyl-L-tyrosine) and L-dopa (3-hydroxy-L-tyrosine) derived from vanillin are used as antihypertensive and antiparkinsonism drugs, respectively.
Furthermore, trimethoprim, which is synthesized from vanillin, is utilized as an antiinfective agent. Carbazochrome and its sodium sulfonate form are hemostatic agents that are frequently used in the medical field. Meanwhile, papaverine, which serves as an antispasmodic, vasodilator, and smooth muscle relaxant medication, is known for its therapeutic potential in certain medical conditions.
Two significant carbamate insecticides, carbofuran (2,3-dihydro-2,2-dimethyl-7-benzofuranyl methylcarbamate) and propoxur (2-isopropoxyphenyl-N-methylcarbamate), which are derived from catechol, have gained traction as agricultural chemicals.
These insecticides have been extensively employed in the field to eradicate pests and have been commercialized under the trade names Furadan and Baygon, respectively, by Bayer.
The chemical compound 4-tert-butylcatechol is synthesized by ring alkylation of catechol. It is highly valued for its utility as a polymerization inhibitor during the manufacturing and storage of monomers like styrene and butadiene.
Reference
- Phenol Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a19_313


