Aminophenols have been gaining significant commercial importance, both as standalone substances and as crucial components in the chemical and dye sectors.
These compounds possess amphoteric properties showing characteristics of weak acids or weak bases, with the basic nature typically prevailing.
Notably, 2-Aminophenol and 4-aminophenol can easily undergo oxidation, a characteristic that contributes to their primary applications as photographic developers. However, 3-aminophenol remains relatively stable when exposed to air and doesn’t oxidize.
Table of Contents
1. Physical Properties of Aminophenols
The basic aminophenol structures exist in three distinct isomeric configurations, determined by the spatial arrangement of the hydroxyl and amino groups adjacent to the benzene ring. These compounds typically solidify into crystalline structures at normal room temperature.
The aminophenol compounds available on the market are commonly impure due to potential contamination by oxidation byproducts. As a result, they might exhibit hues ranging from yellow-brown to pink-purple, with the 2- and 4-aminophenol being more vulnerable to this color alteration compared to the 3-isomer.
1.1. Physical Properties of 2-Aminophenol
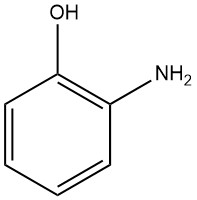
2-Aminophenol, also known as 2-hydroxyaniline or 2-amino-1-hydroxybenzene, has the chemical formula C6H7NO, a molar mass of 109.13 g/mol. When it is crystallized from water or benzene, it forms white orthorhombic bipyramidal needles.
The melting point of these crystals is 174 °C and their density is approximately 1.328 g/cm3 (1.29 g/cm3 is also mentioned in some sources). Under reduced pressure (1.47 kPa), 2-aminophenol sublimates rapidly at 153 °C without decomposition.
Regarding its acid-base properties, the dissociation constants are as follows: pK1 is 4.72 at 21 °C in water, and 4.66 at 25 °C in a solution of 1% ethanol in water; pK2 is 9.66 at 15 °C and 9.71 at 22 °C in water.
The compound can form various salts, including the hydrochloride salt, which appears as needles and has a melting point of 207 °C; the formate salt, which melts at 120 °C; the oxalate salt, which decomposes at 167.5 °C; and the acetate salt, which melts at 150 °C.
2-Aminophenol is very soluble in Acetone, Acetonitrile, Dimethylsulfoxide, Ethanol and Ethyl acetate, soluble in hot water and diethyl ether and slightly soluble in chloroform, benzene, toluene and cold water.
1.2. Physical Properties of 3-Aminophenol

3-Aminophenol also referred to as 3-hydroxyaniline or 3-amino-1-hydroxybenzene, has the chemical formula C6H7NO with a molar mass of 109.13 g/mol. This compound forms white prisms upon crystallization from water or toluene, and its melting point ranges between 122 and 123 °C.
The orthorhombic crystals exhibit a tetramolecular arrangement and possess a density of approximately 1.195 g/cm3 (alternative values of 1.206 and 1.269 have also been reported). When subjected to reduced pressure (1.47 kPa), 3-aminophenol boils at 164 °C with slight decomposition.
Regarding its acid-base properties, the dissociation constants are as follows: pK1 is 4.17 at 21 °C in water, and 4.31 at 25 °C in a solution containing 1% aqueous ethanol; pK2 is 9.87 at 22 °C in water.
3-Aminophenol can form various salts, including the hydrochloride salt, which takes the form of prisms and melts at 229 °C; the hydrobromide salt, also prismatic, with a melting point of 224 °C; the hydroiodide salt, also prismatic, with a melting point of 209 °C; the sulfate salt occurring as plates or needles and melting at 152 °C; and the oxalate salt, with a melting point of 275 °C.
3-Aminophenol is very soluble in Acetone, Acetonitrile, Dimethylsulfoxide, Ethanol, Ethyl acetate and hot water, soluble in cold water and diethyl ether and slightly soluble in chloroform, benzene and toluene.
1.3. Physical Properties of 4-Aminophenol
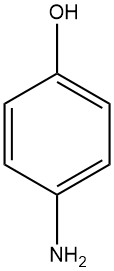
4-Aminophenol, known as 4-hydroxyaniline or 4-amino-1-hydroxybenzene, has a chemical formula of C6H7NO with a molecular weight of 109.13 g/mol. It takes the form of white plates upon crystallization from water and has a melting range of 189 to 190 °C (decomposition occurs).
The crystals exist in two distinct forms. The more stable “α” form (obtained from alcohol, water, or ethyl acetate) has an orthorhombic pyramidal structure with four molecules within each unit cell. It has a density of approximately 1.290 g/cm3 (alternatively, 1.305 g/cm3 is reported).
The less stable “β” form (crystallization from acetone) presents as acicular crystals, which convert to the “α” form over time. The “β” form’s crystals are orthorhombic bipyramidal or pyramidal and exhibit a hexamolecular arrangement.
At reduced pressure (40 Pa), 4-aminophenol sublimes at 110 °C with slight decomposition. Boiling points under various pressures are as follows: 284 °C (101.3 kPa), 174 °C (1.47 kPa), 167 °C (1.07 kPa), 150 °C (0.4 kPa), and 130.2 °C (0.04 kPa), with decomposition usually occurring.
The acid-base dissociation constants for this compound are as follows: pK1 is 5.50 at 21 °C in water, 4.86 at 30 °C in water, and 5.48 in a solution containing 1% aqueous ethanol at 25 °C; pK2 is 10.30 at 22 °C in water and 10.60 at 30 °C in water.
Various salts of 4-aminophenol can be formed, including the hydrochloride salt, which presents as prisms and undergoes decomposition upon reaching 306°C; the hydrosulfate salt, appearing as needles with a melting point of 272°C; the oxalate salt, which melts at 183°C; the acetate salt, melting at 183°C; the chloroacetate salt forms needles with a melting point of 148 °C; and the trichloroacetate salt, in the form of needles with a melting point of 166 °C.
4-Aminophenol is very soluble in dimethylsulfoxide, soluble in acetone, acetonitrile, ethyl acetate and hot water and diethyl ether, slightly soluble in ethanol, diethyl ether, toluene and cold water and insoluble in benzene and chloroform.
2. Chemical Reactions of Aminophenols
Detailed discussions regarding the chemical attributes and responses of aminophenols are extensively documented within established references of the field. A concise overview is provided here.
The presence of an amino group in the benzene ring suppresses the acidity of phenols, with 4-aminophenol exhibiting the most pronounced effect. Aminophenols act as weak bases, forming salts with both organic and inorganic acids.
Aminophenols are ampholytes with no zwitterion structure . They exist as neutral molecules, ammonium cations, or phenolate anions depending on the solution’s pH.

However, deviations from theoretical acid-base titration curves have led to the hypothesis of half-salt complex cations B2+, formed by associating an ammonium cation, B+, with a neutral molecule, B. This phenomenon is especially noticeable in 4-aminophenol and is also demonstrated by other isomers.
Aminophenols are chemically reactive, participating in reactions involving both the aromatic amino group and the phenolic hydroxyl moiety, as well as benzene ring substitution.
Oxidation leads to the development of vibrantly colored polymeric quinoid structures. 2-Aminophenol undergoes an array of cyclization reactions.
2.1. Alkylation of Aminophenols
All mono-, di-, and trimethylated aminophenols can be formed. Monoalkylation occurs upon heating the aminophenol with an appropriate alkyl halide, an alcohol in the presence of Raney nickel, or aldehydes and ketones as alternatives to alcohol.
Direct alkylating of the hydroxyl group to produce methoxyanilines (anisidines) or ethoxyanilines (phenetidines) is intricate due to the amino group’s reactivity, often yielding mixed alkylated products.
Methylation of 3-aminophenol under alkaline conditions yields 3-methoxyaniline, although a more common approach involves amino group protection followed by 3-acetylanilines methylation and subsequent hydrolysis.
Other anisidines and phenetidines are typically produced indirectly by nitro analog reduction:

2.2. Acylation of Aminophenols
Acylation of aminophenols, employing acetic anhydride in alkali or pyridine, acetyl chloride and pyridine in toluene, or ketene in ethanol, generally yields N-acylated products. Excessive reagent use, particularly with 2-aminophenol, leads to O,N-diacylated products.
Aminophenyl carboxylates (O-acylated aminophenols) are typically produced via the reduction of corresponding nitrophenyl carboxylates, a process of particular significance for 4-aminophenol derivatives.
Migration of the acyl group from O to N positions is observed in both 2- and 4-aminophenol acylated products.
An exemple of rearrangemant is the formation of 2-hydroxyphenyl carbamate by slowly treating 2-Aminophenyl ethyl carbonate in dilute acid. The para derivatives don’t show any transformation.

2.3. Diazonium Salt Formation
Aminophenols’ aromatic amino groups can be converted to diazonium salts by treatment with sodium nitrite under acidic aqueous conditions. Challenges may arise with easily oxidizable or sparingly soluble aminophenols.
Crystalline diazonium salts are isolated using hydrochloride or sulfate forms of the relevant aminophenol under anhydrous conditions. These diazo derivatives find widespread use in the dye industry.
2.4. Aminophenols Cyclization Reactions
Due to the close proximity of the amino and hydroxyl groups in 2-aminophenol, this isomer is especially susceptible to cyclization and condensation reactions. Oxidation by iron(III) chloride, enzymes, light, or autoxidation on silica thin-layer plates yields 2-aminophenoxazin-3-one (4).
Subsequent oxidation with iron(III) cyanide or heating with potassium hydroxide in ethanol generates a five-ring structure, triphenoxdioxazine (benzoxazinophenoxazine) (5).

2-Aminophenol and its derivatives serve as valuable starting materials for synthesizing phenoxazones, phenoxazines, benzoxazoles, and thiobenzoxazoles, with most of these reactions involving heating at 200 – 300 °C with an appropriate catalyst.
2.5. Condensation Reactions
Substituted diphenylamines or diphenyl ethers are obtained from aminophenols through the elimination of ammonia or hydrogen chloride.
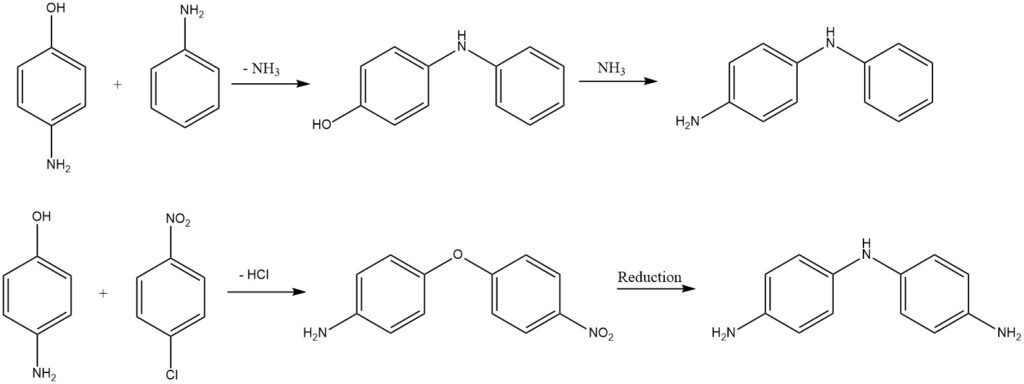
2.6. Reactions of the Benzene Ring
Both amino and hydroxyl groups function as electron-donating moieties, leading to the formation of various substituted derivatives. Controlled interactions between amino-phenols and chlorine or bromine in glacial acetic acid yield a spectrum of halogenated products.
Concentrated sulfuric acid or oleum, with or without heat, generates aromatic sulfonic acids. The sulfonic acid group predominantly enters the ortho or para positions relative to the hydroxyl group. Further treatment with oleum results in disulfonated compounds.
Carboxylation of m-aminophenol yields p-aminosalicylic acid.
3. Production of Aminophenols
Aminophenols are synthesized by two primary routes: reduction of nitrophenols or substitution reactions. Reduction can be achieved using iron or hydrogen in the presence of a catalyst. Today, the latter method is the preferred choice for producing 2- and 4-aminophenol.
3.1. Reduction of Nitro Compounds
3.1.1. Iron Reduction
The reduction of nitrophenols using iron turnings occurs in a mildly acidic solution or suspension. Following reduction, the iron-iron oxide sludge is separated from the solution. To enhance water solubility, sodium hydroxide is added, producing sodium aminophenolate.
Due to susceptibility to oxidation in aqueous solution, various purification methods are advised. Subsequently, aminophenols are precipitated from acidic solutions by base neutralization, while reducing agents are often incorporated.
Nonetheless, the reduction of 2-nitrophenol with iron generates insoluble color lakes as byproducts, decreasing the yield. As a result, the industrial significance of iron reduction for 2-nitrophenol is limited.
3.1.2. Catalytic Reduction
Catalytic reduction primarily transpires in solution, emulsion, or suspension within autoclaves or pressurized vessels. Following catalyst addition, pressurized hydrogen is introduced into the vessel. Preferred solvents encompass water and methanol, with water being supplemented by alkali hydroxide, alkali carbonate, or acid.
Catalysts such as nickel – particularly Raney nickel – or supported precious metals such as platinum or palladium on activated carbon, or their oxides, are employed.
Adding immiscible organic solvents to water can extend catalyst life, decrease consumption, and enhance product quality.
Typically, a hydrogen pressure of 2 MPa is optimal, although atmospheric or higher pressures up to 6 MPa can be used. Reaction temperatures remain below 100 – 110 °C.
3.1.3. Nitrobenzene Reduction
Acidic medium reduction of nitrobenzene yields 2- and 4-aminophenol via rearrangement of the intermediate phenylhydroxylamine. The main product is 4-aminophenol, alongside byproducts 2-aminophenol, aniline, and 4,4′-diaminodiphenyl ether.
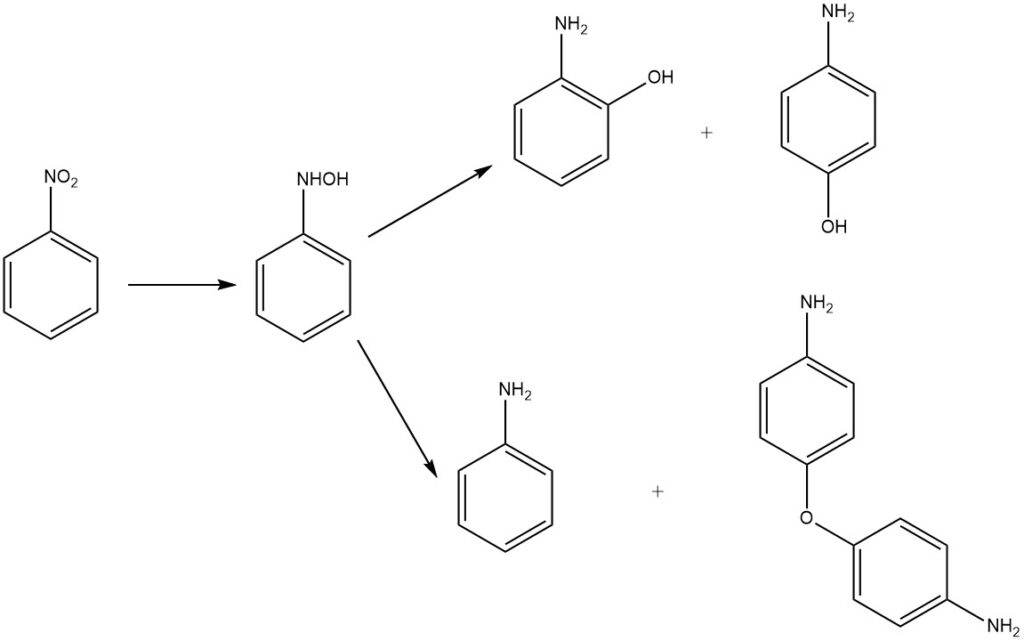
Historically, metals in dilute sulfuric acid served as reducing agents. Presently, hydrogen and precious-metal catalysts such as palladium or platinum, are employed. Additional catalysts include molybdenum and platinum sulfide and a platinum – ruthenium mixed catalyst, often in oxide form or supported on activated carbon.
Dilute aqueous mineral acid acts as the reaction medium, and a two-step process involving Raney copper selectively reduces nitrobenzene to phenylhydroxylamine, which rearranges to 4-aminophenol upon dilute sulfuric acid addition.
Wetting agents, particularly quaternary ammonium salts with sizable alkyl groups, increase aminophenol yield. Typically, the reaction occurs below 100 °C and may use atmospheric or higher pressure. Hydrogen addition occurs as consumed, and inert organic solvents can boost yields.
In an alternative method, nitrobenzene reduction stops at 88%, creating a two-phase mixture containing unreacted nitrobenzene and the precious-metal catalyst. Phase separation suffices for workup, with the nitrobenzene phase recycled.
The aqueous sulfuric acid phase contains 4-aminophenol and aniline which is stripped after neutralization. Purification and crystallization yield 4-aminophenol.
3.1.4. Electrolytic Reduction
Electrolytic reduction offers a less polluting alternative to metal-acid systems, yet industrial implementation remains limited. Electrolysis of nitrobenzene, phenylhydroxylamine, or azoxybenzene in acid solutions yields specifically 4-aminophenol.
3.2. Substitution
The substitution of amino or hydroxyl groups in place of various other groups is not of significant industrial importance for producing 2- and 4-aminophenol. However, this type of reaction finds utility in synthesizing derivatives of 2- and 4-aminophenol.
Conversely, the straightforward reduction route does not readily yield 3-aminophenol. Its production primarily involves the reaction between 3-aminobenzenesulfonic acid and sodium hydroxide or the reaction of resorcinol with ammonia.
Substitution of the sulfonic acid group in 3-aminobenzenesulfonic acid is achieved by a caustic soda fusion process (lasting 5 to 6 hours at 240 to 245 °C), followed by vacuum distillation to purify the product.
An alternative method involves the reaction of resorcinol with ammonia, potentially in the presence of diammonium phosphate and arsenic pentoxide or ammonium sulfite, resulting in the formation of 3-aminophenol.
Additionally, 3-aminophenol can be obtained by the hydrolysis of 3-aminoaniline.
3.3. Purification of Aminophenols
Aminophenols are purified by sublimation at elevated temperatures and reduced pressure.
For 3-aminophenol, vacuum distillation is a suitable purification method, where the addition of sulfur dioxide during distillation or the collection of the distillate under a layer of an inert liquid of lower density, such as water, helps obtain a colorless product.
An alternative purification approach involves treating the aqueous solutions of aminophenols with activated carbon. During this process, the inclusion of substances like sodium sulfite, sodium dithionite, or disodium ethylenediaminotetraacetate serves to enhance the quality and stability of the products while chelating heavy-metal ions that could facilitate oxidation.
The addition of sodium dithionite, hydrazine, or sodium hydrosulfite is also recommended during the precipitation or crystallization of aminophenols.
For aminophenols, particularly 4-aminophenol produced by catalytic reduction, contaminants can often be diminished or eliminated by employing various procedures. These methods include treatment with 2-propanol, aliphatic, cycloaliphatic, or aromatic ketones, aromatic amines, toluene, or low-molecular-weight alkyl acetates, as well as the use of phosphoric acid, hydroxyacetic acid, hydroxypropionic acid, or citric acid.
Extraction with methylene chloride, chloroform, or nitrobenzene can also be effective in mitigating or removing contaminants.
4. Uses of Aminophenols
Both 2-aminophenol and 4-aminophenol are potent reducing agents, rendering them valuable as photographic developers, recognized by commercial names such as Atomal and Ortol for 2-aminophenol, and Activol, Azol, Certinal, Citol, Paranol, Rodinal, Unal, and Ursol P for 4-aminophenol.
These substances find application either individually or in conjunction with hydroquinone. The oxalate form of 4-aminophenol is marketed under the trade name Kodelon.
The versatility of aminophenols makes them essential intermediates in the synthesis of nearly all types of stains and dyes. Moreover, 2-aminophenol is specifically employed for enhancing the hues of leather, fur, and hair, ranging from gray and brown shades to yellowish-brown tones.
For diverse purposes, 3-aminophenol serves as a hair colorant and a coupling agent in hair dye formulations. Meanwhile, 4-aminophenol is integral to pharmaceutical synthesis, contributing to the production of wood stains that bestow a rose-like tint to timber, and acting as a dyeing agent for fur and feathers.
Given the close proximity of the amino and hydroxyl groups on the benzene ring, along with their predisposition for condensation with appropriate reactants, 2-aminophenol is a key precursor in the synthesis of heterocyclic systems like oxyquinolines, phenoxamines, and benzoxazoles which are used as inflammation inhibitors.
With respect to 3-aminophenol, its applications encompass the stabilization of chlorine-containing thermoplastics although its principal role is being an intermediate for producing 4-amino-2-hydroxybenzoic acid, a tuberculostatic agent.
Similarly, derivatives of nitrogen-substituted 4-aminophenols have long been recognized for their antipyretic and analgesic properties, contributing significantly to the utilization of 4-aminophenol.
5. Toxicology of Aminophenols
Aminophenols are classified as irritants. Their toxicity rating ranges from slight to moderate; however, prolonged or repetitive exposure can lead to various adverse effects, including general itching, skin sensitization, dermatitis, and allergic responses.
Exposure to 2- and 4-aminophenol can lead to the spontaneous production of immunogenic conjugates. Another potential concern is the formation of methemoglobin, resulting in cyanosis.
Inhalation of 4-aminophenol may trigger this reaction and also lead to bronchial asthma. Notably, 3-aminophenol poses a lesser hazard compared to the other isomers. The irritation caused by sulfonated derivatives is milder compared to unsulfonated compounds.
2-Aminophenol has neuroactive properties, inducing spike discharges when introduced into the cerebroventricle of rats. On the other hand, 4-aminophenol is nephrotoxic and significantly inhibits proximal tubular function.
In rat kidney mitochondria, respiration, oxidative phosphorylation, and ATPase activity are inhibited by 4-aminophenol. While 3-aminophenol is not as hazardous, both 2-aminophenol and 4-aminophenol exhibit teratogenic effects in hamsters.
The impact of 4-aminophenol extends to DNA synthesis inhibition and alteration of DNA structure in human lymphoblasts. Additionally, aminophenols have been identified as genotoxic agents, as evidenced by the induction of sister chromatid exchanges and micronucleus tests.
Clearly, cautious handling of these compounds is essential, and extended exposure should be minimized. Swift removal of contaminated clothing and thorough washing of the affected area with running water for a minimum of 10 minutes are recommended procedures.
References
- Aminophenols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_099
- Aminophenols; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/abs/10.1002/0471238961.0113091413092003.a01.pub2