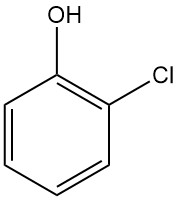
2-Chlorophenol, also known as ortho-chlorophenol, is an aromatic compound with the chemical formula C6H4ClOH. It is a liquid with a strong, unpleasant odor.
Table of Contents
1. Physical Properties of 2-Chlorophenol
2-Chlorophenol is a colorless to amber liquid (commercial samples may have a yellow tint) with a strong, penetrating odor. It is soluble in water and highly soluble in alcohols, ethers, and alkali solutions because it is a weak acid. Important physical properties of 2-chlorophenol are shown in Table 1.
| Property | Value |
|---|---|
| CAS number | [95-57-8] |
| Molecular weight | 128.56 g/mol |
| Melting point | 8.7 °C |
| Boiling point | 174.5 °C |
| pKa | 8.52 |
| Density at 20 °C | 1.263 |
| Specific heat at 20 °C, J.mol-1K-1 | 177 |
| Heat of formation at 50 °C, kJ/mol | 185 |
| Vapor Pressure at 25°C | 2.53 mmHg |
| Viscosity at 50 °C, mPa.s | 2 |
| Refractive Index | 1.55 |
| Solubility in water at 20 °C | 28.5 g/L |
| Flash point (closed cup) | 121 °C |
2. Chemical Reactions of 2-Chloophenol
2-Chlorophenol is a valuable starting material in various chemical syntheses due to the reactivity of both the hydroxyl group and the aromatic ring. It participates in both electrophilic and nucleophilic substitutions and oxidation reactions.
2.1. Reactivity of the hydroxyl group
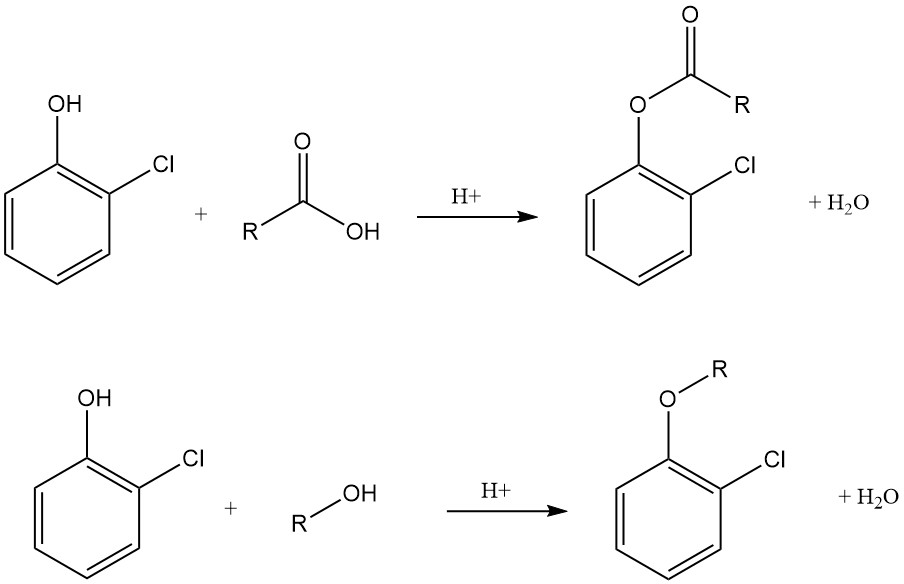
Compared to phenol, 2-chlorophenol is more acidic, forming stable and water-soluble salts with alkali and alkaline-earth metal hydroxides, carbonates, or amines. It readily forms esters with organic and inorganic acids, including the widely used 2-chlorophenyl phosphate, carbamate, chloroformate, carbonate, and laurate.
2-Chlorophenol undergoes O-alkylation to generate a diverse range of ethers derived from aliphatic, aromatic, and heterocyclic compounds.
2.2. Electrophilic aromatic substitution
The presence of a chlorine atom on the aromatic ring of 2-chlorophenol makes electrophilic substitution reactions easier. Therefore, it can participate in standard reactions such as alkylation, acylation, nitration, sulfonation, halogenation, carboxylation, and condensation with aldehydes like formaldehyde, chloral, and glyoxylic acid.
2.3. Nucleophilic aromatic substitution
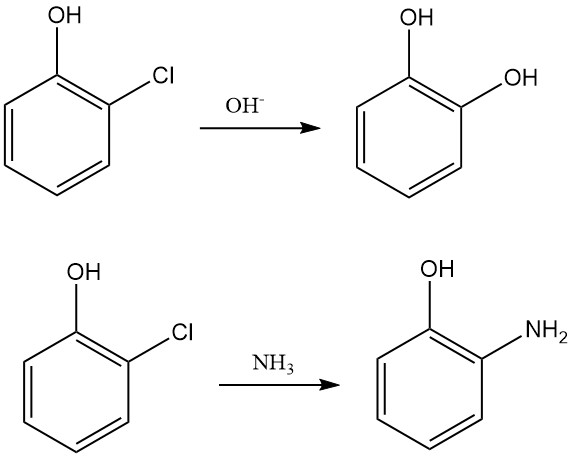
Nucleophilic substitution of the chlorine atom in 2-chlorophenol is still used in chemical synthesis, mainly to produce pyrocatechol by reacting with potassium or sodium hydroxide. 2-Chlorophenol also reacts with ammonia to form 2-aminophenol.
This reaction type is particularly useful for producing various substituted diphenyl ethers, which are efficient herbicides.
2-Chlorophenol can be transformed into polychlorinated alicyclic ketones, like “hexachlorophenol” (a pesticide), by reaction with chlorine in aqueous acetic acid. In the presence of an acidic catalyst like sulfuric acid, hexachlorophenol hydrolyzes to chloranil.
2.4. Oxidation
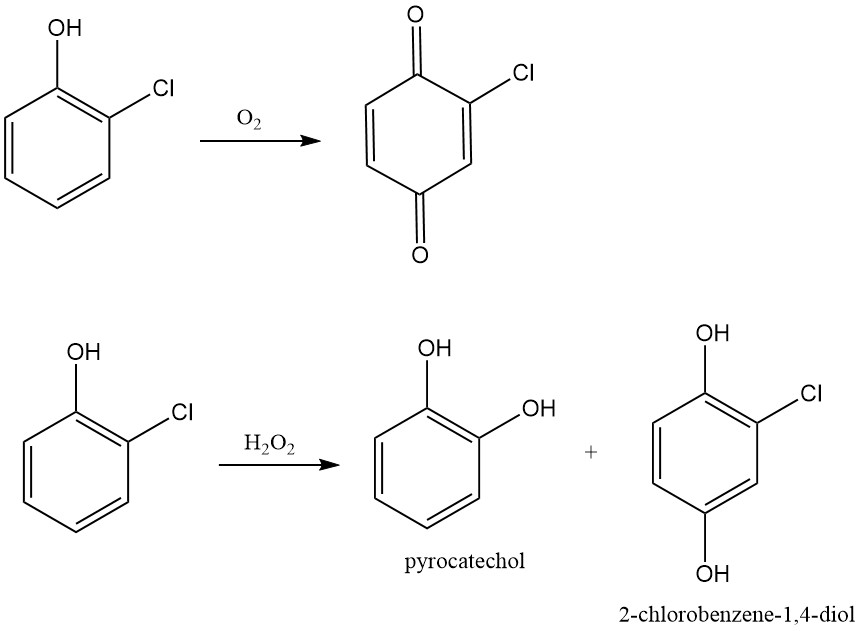
Similar to other phenols, 2-chlorophenol undergoes oxidation. Air oxidation leads to the formation of 2-chloroquinone, while hydrogen peroxide oxidation produces the corresponding chlorinated diphenols. Peroxide-mediated oxidation of 2-chlorophenol using perpropionic acid in benzene at 80 °C gives chlorodiphenols (3-chlorocatechol and chlorohydroquinone) with an 85% yield.
3. Production of 2-Chlorophenol
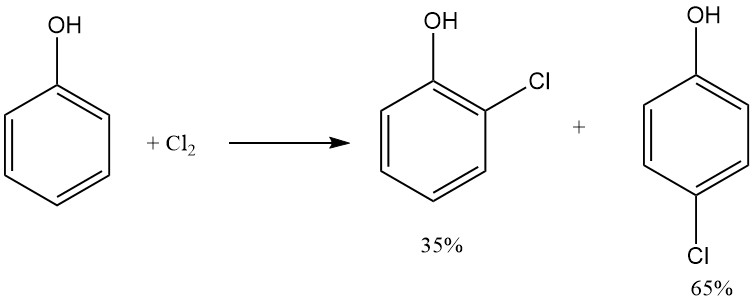
2-Chlorophenol is produced by the direct chlorination of melted phenol using chlorine gas. This process yields a mixture of ortho and para monochlorophenol, with 2-chlorophenol at 35% and 4-chlorophenol at 65%, and is almost not dependent on temperature.
To increase the yield of 2-chlorophenol, the following modifications can be made to the chlorination reagent or the reaction medium:
- Solvent: Aprotic non-polar solvents like hexane, carbon tetrachloride, and dichloroethane promote the formation of 2-chlorophenol.
- Temperature and Concentration: Higher temperatures and lower phenol concentrations favor the ortho isomer.
- Chlorinating Agent: Certain reagents like diisopropylamine (0.01 wt% in perchloroethylene, 110 °C) yield a very high o/p ratio (15).
- The chlorination of phenol using sodium hypochlorite (pH 10), tert-butyl hypochlorite, alkali phenolates in anhydrous media, and chlorine in acetic acid with acetic anhydride favors the formation of 2-chlorophenol.
4. Uses of 2-Chlorophenol
2-chlorophenol has limited current uses due to the availability of safer and more effective alternatives. However, it has some historical and potential applications.
In the past, 2-chlorophenol was used as a disinfectant due to its bactericidal and germicidal properties. However, its potential health hazards led to its discontinuation in most disinfectant applications.
The primary use of 2-chlorophenol today is as an intermediate in the production of other chemicals. It serves as a starting material for the synthesis of various compounds, including:
- Herbicides: Certain phenoxy herbicides, like dichlorophenoxyacetic acid (2,4-D), can be produced using 2-chlorophenol as a precursor.
- Dyes: Some dyes, particularly azo dyes, use 2-chlorophenol as a starting material in their synthesis.
- Higher chlorinated phenols, catechol, and profenofos.
Research is ongoing to explore potential new applications for 2-chlorophenol, particularly in areas like the development of new fungicides or insecticides, the production of bio-based polymers, and the extraction of sulfur and nitrogen compounds from coal.
5. Toxicology of 2-Chlorophenol
2-chlorophenol exhibits significant toxicity, causing various adverse effects upon exposure.
High doses of 2-chlorophenol induce convulsions, often triggered by external stimuli like touch or sound. Additional symptoms include agitation, rapid breathing (polypnea), muscle weakness, respiratory distress (dyspnea), and coma, potentially leading to death. Repeated exposure to toxic levels primarily damages the liver and kidneys.
For rats, the oral and percutaneous LD50 values are 670 mg/kg and 950 mg/kg, respectively.
Research on the elimination and metabolism of 2-chlorophenol remains inconclusive. Studies suggest that dogs primarily excrete it in the urine, both in free and conjugated forms (87%). Conversely, rabbits appear to convert 2-chlorophenol to chlorobenzene before excretion.
Currently, there are no reported cases of chronic health effects in humans exposed to low doses of 2-chlorophenol.
References
- Chlorophenols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a07_001.pub2
- https://pubchem.ncbi.nlm.nih.gov/compound/2-Chlorophenol