Acetyl chloride: Properties, Reactions, Production and Uses
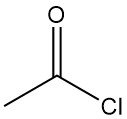
What is acetyl chloride?
Acetyl chloride, also known as ethanoyl chloride, is an organic compound with the molecular formula CH3COCl. It is a colorless corrosive and strongly irritating liquid with a suffocating odor.
Concentrations of acetyl chloride as low as 0.5 ppm induce lacrimation and cause burning of the eyes, nasal passages, and throat. Acetyl chloride is toxic because it reacts readily with hydroxyl, sulfhydryl, and amino groups, producing modifications that inhibit the activity of essential enzymes in living tissues.
Table of Contents
1. Physical properties of acetyl chloride
Acetyl chloride is a colorless, corrosive liquid. The main physical properties of acetyl chloride are summarized in Table 1.
| Property | Value |
|---|---|
| CAS Registry Number | 75-36-5 |
| Molecular formula | C2H3OCl |
| Molar mass (g/mol) | 78.50 |
| Freezing point (°C) | -112.0 |
| Boiling point (°C, 101.3 kPa) | 50.2 |
| Density (g/mL) at 4 °C | 1.1358 |
| Density (g/mL) at 20 °C | 1.1051 |
| Density (g/mL) at 25 °C | 1.0982 |
| Standard enthalpy of formation (ΔHf, kJ/mol) | -243.93 |
| Enthalpy of vaporization at boiling point (ΔHv, kJ/g) | 0.36459 |
| Refractive index (nD20) | 1.38976 |
2. Chemical reactions of acetyl chloride
Acetyl chloride undergoes a wide range of chemical reactions and is used in organic synthesis for:
- Electrophilic acetylation of arenes, alkenes, alkynes, saturated alkanes, organometallics, and enolates (on C or O)
- Cleavage of ethers
- Esterification of sterically unhindered or acid-sensitive alcohols
- Generation of solutions of anhydrous hydrogen chloride in methanol
- As a dehydrating agent
- As a solvent for organometallic reactions
- For deoxygenation of sulfoxides
- As a scavenger for chlorine and bromine
- As a source of ketene
- For nucleophilic acetylation
In inert solvents such as carbon disulfide or petroleum ether, acetyl chloride reacts with aromatic hydrocarbons and olefins to produce ketones. These reactions are catalyzed by Lewis acids, with catalytic activity increasing in the order:
ZnCl2 < BiCl3 < TeCl4 < TiCl4 < SnCl4 < TeCl2 < FeCl3 < SbCl5 < AlCl3
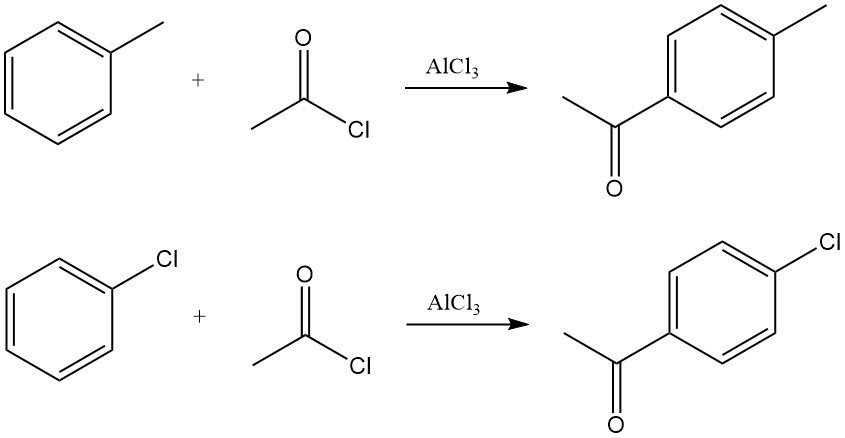
Arenes react with acetyl chloride in the presence of Lewis acids, typically aluminum chloride, to yield aryl methyl ketones. The reaction proceeds through generation of the acylium ion, which undergoes electrophilic aromatic substitution.
For example, acetylation of toluene gives predominantly p-methylacetophenone, while acetylation of chlorobenzene yields p-chloroacetophenone with high selectivity.
Alkenes react with acetyl chloride under Friedel–Crafts conditions to form mixtures of chloroalkyl methyl ketones and related products. Electron-deficient alkenes such as ethylene and allyl chloride give good yields, whereas higher alkenes often undergo rearrangements. For example, reaction of acetyl chloride with cyclohexene using AlCl3 as catalyst yields 2-chlorocyclohexyl methyl ketone and 4-chlorocyclohexyl methyl ketone.

Alkynes undergo electrophilic acetylation under similar conditions. Acetylene treated with acetyl chloride and aluminum chloride produces β-chlorovinyl methyl ketone, while higher alkynes give ketonic products in moderate to high yields.
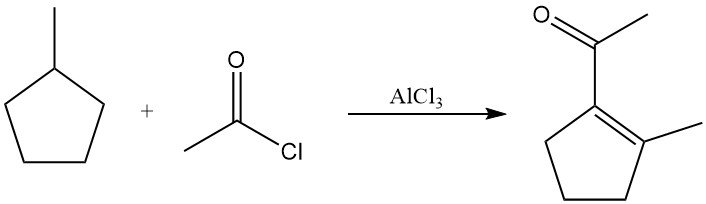
In the presence of acetyl chloride and aluminum chloride at elevated temperature, saturated alkanes undergo dehydrogenation to alkenes followed by acetylation. The products are typically vinyl methyl ketones or saturated alkyl methyl ketones. Methylcyclopentane, for example, yiels 1-acetyl-2-methylcyclopentene in significant yield.
Acetyl chloride reacts with a wide range of organometallic compounds, including Grignard, organolithium, organozinc, and organocuprate reagents, to give methyl ketones. Transition-metal catalysts such as palladium or copper salts are often employed to improve selectivity and yields.
Metal enolates react with acetyl chloride to form β-diketones. The reaction may also give competing O-acetylated products depending on the metal counterion. Use of zinc or copper enolates improves selectivity for C-acetylation. Related transformations include acetylation of silyl ketene acetals and enamines, providing access to functionalized esters and ketones.
Ketones and β-keto esters can be converted into enol acetates using acetyl chloride. The reaction may proceed through pre-formed enolates or silyl enol ethers. For example, steroidal ketones can be converted into conjugated enol acetates by treatment with acetyl chloride in the presence of amine bases.
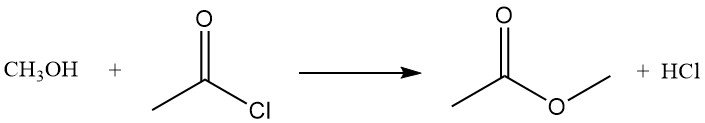
Acetyl chloride reacts with alcohols to give esters or alkyl chlorides. Primary alcohols usually form esters, while secondary and tertiary alcohols often give chlorides via substitution. The reaction of methanol with acetyl chloride produces methyl acetate and hydrogen chloride, and is used as a method to prepare solutions of anhydrous hydrogen chloride in methanol.
Cyclic and acyclic ethers can be cleaved by acetyl chloride in the presence of halide salts or Lewis acids. For example, tetrahydrofuran reacts with acetyl chloride and sodium iodide to form 4-iodobutyl acetate and in the presence of a Lewis acid such as ZnCl2 to form chlorobutyl acetate in about 76% yield. This property makes acetyl chloride useful in deprotection of ether protecting groups.

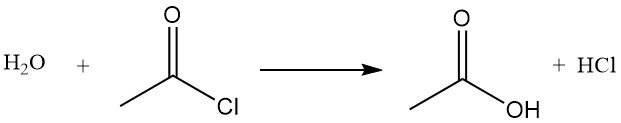
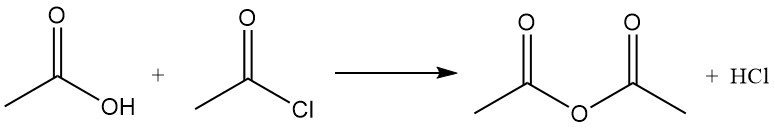
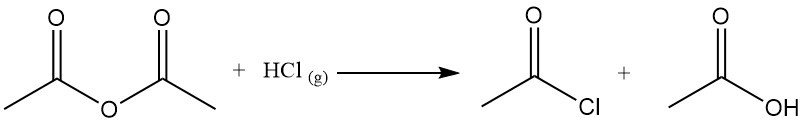
Acetyl chloride acts as a dehydrating agent by reacting with water to form acetic acid and hydrogen chloride. It promotes cyclization of dicarboxylic acids to anhydrides, keto acids to lactones, and nitro compounds to nitrile oxides. It is also used in the conversion of hydroperoxides to unsaturated ketones. For example, It reacts with acetic acid to produce acetic anhydride.
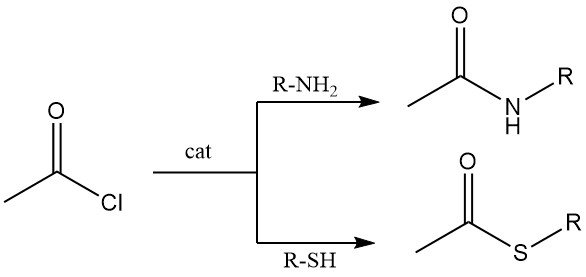
Primary and secondary amines react with acetyl chloride to yield acetamides. Under Schotten–Baumann conditions (aqueous base), N-acetylation is efficient, though hydrolysis of acetyl chloride may compete.
Tertiary amines yield acetylammonium salts, which can decompose to ketenes or undergo von Braun fragmentation. Related reactions include N-acetylation of imines to enamides and conversion of urethanes to imides.
Thiols react with acetyl chloride to give thioesters. Both aliphatic and aromatic thiols undergo this transformation, typically in the presence of cobalt chloride as a catalyst.
Acetyl chloride forms reversible adducts with aldehydes and ketones in the presence of Lewis acids, producing α-chloroalkyl acetates. For example, acetone reacts with acetyl chloride and zinc chloride at low temperature to form the corresponding adduct in good yield.
Sulfoxides are reduced to sulfides by acetyl chloride in the presence of tin(II) chloride. The reaction proceeds under mild conditions and is compatible with sensitive functional groups, including cephalosporin derivatives.
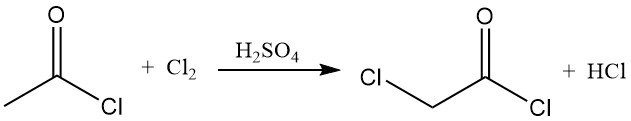
Acetyl chloride reacts with chlorine and bromine to form haloacetyl chlorides. In the presence of sulfuric acid, chlorine is efficiently converted to chloroacetyl chloride, which is an important synthetic intermediate.
Reactions of acetyl chloride with tertiary amines such as triethylamine generates ketene in situ. The ketene can be trapped by enolates, enamines, or imines to form acetoacetic esters, cyclobutanones, or diketene adducts.
In the presence of samarium(II) iodide, acetyl chloride behaves as a nucleophilic acetyl anion equivalent, giving acyloin products from ketones.
Reaction of acetyl chloride with hydrogen peroxide produces peroxyacetic acid and acetyl peroxide, the latter being a highly explosive compound.
In the presence of fatty acids, it forms mixed acetic–alkylcarboxylic anhydrides or acyl chlorides, both of which can be used in esterification reactions. For example, lauric acid reacts with acetyl chloride to form the corresponding derivatives.
Acetyl chloride undergoes reduction with organometallic reagents such as lithium aluminum hydride (LiAlH4). In the presence of tert-butyl alcohol, LiAlH4 is converted to lithium tri-tert-butoxyaluminum hydride, which selectively reduces acetyl chloride to acetaldehyde. Triphenyltin hydride also reduces acetyl chloride.
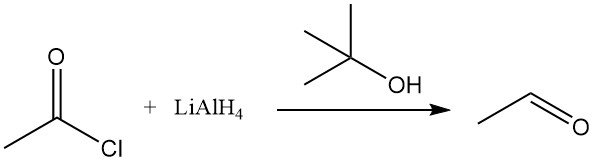
Catalytic hydrogenation of acetyl chloride by the Rosenmund method is inefficient, but reduction of acetic anhydride to ethylidene diacetate is possible in the presence of acetyl chloride over palladium complexes. Rhodium trichloride, methyl iodide, and triphenylphosphine form a complex that is active in reducing acetyl chloride.
3. Production of acetyl chloride
Acetyl chloride is produced commercially in Europe and the Far East. In the United States, production is limited and mainly directed to on-site use, particularly for pharmaceutical acetylation.
Acetyl chloride was first described in the 1850s. It was obtained by distilling anhydrous sodium acetate with phosphorus oxychloride.
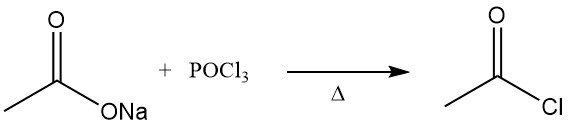
The former method of manufacturing acetyl chloride was the reaction of thionyl chloride, SOCl2, with gray acetate of lime. This process has been largely replaced by the reaction of sodium acetate or acetic acid with phosphorus trichloride, PCl3. A similar route has continued to be applied in the Soviet Union. Both methods are relatively expensive.
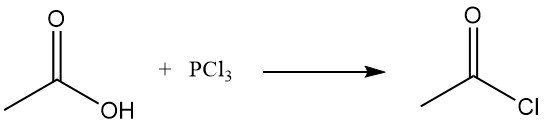
The carbonylation of methyl chloride with carbon monoxide to produce acetyl chloride has been described in several patents. These processes use catalysts based on rhodium, palladium, or iridium complexes in combination with iodo compounds and either phosphonium iodides or phosphine oxides.
For example, one reported reaction yielded a 56% conversion to acetyl chloride at a temperature of 453 K and a pressure of 8360 kPa. These reactions are feasible due to the availability of corrosion-resistant alloys. Industrial application of this method has not been confirmed.
Alternative preparations include the reaction of acetic acid with chlorinated ethylenes in the presence of ferric chloride, combination of benzyl chloride with acetic acid (85% yield), conversion of ethylidene dichloride (91% yield), and decomposition of ethyl acetate by phosgene to form acetyl chloride and ethyl chloride. The high cost of raw materials and equipment makes the phosgene route unviable.
Chlorination of acetic acid to monochloroacetic acid also produces acetyl chloride as a by-product. Because recovery is costly, acetyl chloride is usually recycled to monochloroacetic acid. A patented method describes scrubbing acetyl chloride and hydrogen chloride mixtures with sulfuric acid to form acetyl sulfate.
A U.S. patent reports an improved yield of acetyl chloride from the reaction of acetic anhydride with hydrogen chloride. In this process, acetyl chloride and part of the acetic acid by-product are continuously removed from the mixture, while the remaining components are recycled to the reactor for further reaction.
4. Uses of acetyl chloride
A small quantity of acetyl chloride is consumed during the start-up phase of acetic acid chlorination to monochloroacetic acid. Once initiated, the acetyl chloride formed as a by-product sustains the catalytic effect.
Acetyl chloride is an effective acetylating agent. It is used in the synthesis of aspirin, acetaminophen, acetanilide, and acetophenone. It is also used in the preparation of liquid crystal compositions required for optical display and memory devices.
Acetyl chloride can be used to replace acetic anhydride or acetic acid as a highly reactive acetylation reagent when reactions are difficult or slow. This makes acetyl chloride particularly useful for preparing specialized polymers that can chelate with metal ions, such as copper, resulting in improved electrical and magnetic properties.
Anthralin is acetylated with acetyl chloride in toluene using pyridine as a catalyst to produce 1,8-dihydroxy-10-acetylanthrone, an intermediate in medications for skin conditions including warts, psoriasis, and acne. Sugar esters can be synthesized under anhydrous conditions with acetyl chloride in a similar manner.
Acetyl chloride has been used in analytical chemistry for hydroxyl group determination, but this method has been largely replaced by spectroscopic techniques. It is still used for the preparation of phenol derivatives without strong acid catalysts, and for acetylating primary and secondary amines.
Acetyl chloride can be substituted for acetic anhydride in many acetylation reactions. Unlike the anhydride, it does not require a mineral acid catalyst. Despite its higher cost, acetyl chloride is widely used where convenience outweighs expense. Reduction in production cost would make acetyl chloride a strong alternative to acetic anhydride in large-scale processes.
5. Toxicology and safety
Acetyl chloride produces suffocating fumes with a sharp, irritating odor. It is highly toxic. Its flammability and vigorous reactivity with water and alkalis require strict precautions during handling.
Adequate ventilation is necessary to remove vapors from working areas. Personnel should use impervious protective clothing to prevent exposure. Bulk containers must be stored in cool, dry locations, isolated from flammable and noncorrosive substances.
References
1. Wagner, F.S., Jr. (2002). Acetyl Chloride. In Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc (Ed.). https://doi.org/10.1002/0471238961.0103052023010714.a04.pub2
2. Pearlman, B.A. (2001). Acetyl Chloride. In Encyclopedia of Reagents for Organic Synthesis, (Ed.). https://doi.org/10.1002/047084289X.ra025
3. Le Berre, C., Serp, P., Kalck, P. and Torrence, G.P. (2014). Acetic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). https://doi.org/10.1002/14356007.a01_045.pub3
