
Allyl chloride, also known as 3-chloropropene, is an organic compound with the chemical formula C3H5Cl. It is a colorless to pale yellow liquid that holds significance in the field of organic chemistry due to its reactivity and industrial applications.
It was first produced in 1857 by Auguste Cahours and August Wilhelm Hofmann by reacting phosphorus chloride with allyl alcohol. The name “allyl” is derived from the Latin word “allium,” which means “garlic.” Allyl chloride has a characteristic garlic odor that can be detected even in small amounts.
In the late 1930s, IG Farbenindustrie and the Shell Development Company developed a process for producing allyl chloride on a large scale by chlorinating propene at high temperatures. This process allowed for high yields of allyl chloride and was quickly adopted by other companies, including Dow, Solvay, and Asahi-Kashima.
Allyl chloride is used in a variety of applications, including the production of epichlorohydrin, glycerin, and pesticides. It is also used as a monomer in the production of plastics and resins.
Table of Contents
1. Physical Properties of Allyl Chloride
Allyl chloride is a colorless, mobile liquid with a penetrating, pungent odor. It has a molecular weight of 76.53 and a melting point of -134 degrees Celsius. Its boiling point at 101.3 kPa is 44.4 degrees Celsius. Its refractive index at 25 degrees Celsius is 1.413.
The table below shows the temperature dependence of the physical properties of allyl chloride
| Temperature (°C) | Density (g/cm³) | Specific heat capacity (kJ kg⁻¹ K⁻¹) | Viscosity (mPa s) | Surface tension (mN/m) |
|---|---|---|---|---|
| 10 | 0.950 | 1.633 | 368 | 28.2 |
| 15 | 0.944 | 1.666 | 347 | 27.4 |
| 20 | 0.938 | 1.700 | 336 | 26.7 |
| 25 | 0.931 | 1.733 | 315 | 25.9 |
| 30 | 0.925 | 1.771 | 307 | 25.2 |
| 40 | 0.911 | 1.816 | 282 | 24.4 |
| 50 | 0.898 | 1.868 | 257 | 23.7 |
The vapor pressure of allyl chloride can be calculated using the following equation:
log(p) = 19.1403 – 2098 / T – 4.2114×log(T)
where p is the vapor pressure in kPa and T is the temperature in Kelvin.
Allyl chloride is miscible with most solvents in general use, such as octane, toluene, and acetone. The solubility of allyl chloride in water at 20 degrees Celsius is 0.36%, and the solubility of water in allyl chloride is 0.08%.
The azeotropic data for allyl chloride are shown in Table 2.
| Component | bp at 101.3 kPa, °C | Allyl chloride, mass fraction, % |
|---|---|---|
| Water | 43 | 97.8 |
| Methanol | 40 | 90 |
| Ethanol | 44 | 95 |
| 2-Propanol | 45 | 98 |
| Formic acid | 45 | 92.5 |
2. Chemical Reactions of Allyl Chloride
Allyl chloride is a very reactive compound that undergoes a variety of addition, polymerization, and substitution reactions. The chlorine atom in allyl chloride can also be easily replaced with other groups. This makes allyl chloride a versatile starting material for the synthesis of a wide range of compounds.
2.1. Addition Reactions
Allyl chloride can add to a variety of compounds, including oxygen, halogens, hydrogen halides, silanes, boranes, carboranes, and phosphorus trichloride. These reactions can be used to prepare a variety of allyl derivatives.
For example, when allyl chloride reacts with oxygen in the liquid phase at ca. 120 °C and in the presence of metal acetates or hydrogen peroxide, it yields glycerol monochlorohydrin.
When allyl chloride reacts with halogens, it yields the corresponding trihalogeno compounds. The reaction with hypochlorous acid, which yields 2,3- and 1,3-glycerol dichlorohydrins (which are then dehydrochlorinated with alkali to give epichlorohydrin), is of great industrial importance.
Allyl chloride reacts with hydrogen halides to form 1,2-dihalogeno compounds. In the presence of peroxides, the reaction with hydrobromic acid yields 1-bromo-3chloro-propane (Kharasch effect), but in highly concentrated hydrogen peroxide solution, 1,2-dibromo-3-chloropropane is formed.
Addition reactions of silanes, boranes, carboranes, and phosphorus trichloride, as well as cycloadditions of allyl cations with alkenes, are also known. Allyl chloride polymerizes with sulfur dioxide to form polysulfones.
2.2. Polymerization
Allyl chloride can polymerize to form poly(allyl chloride), a hard, colorless resin. This resin is used in a variety of applications, including paints, adhesives, and sealants.
2.3. Substitution Reactions
The chlorine atom in allyl chloride can be easily replaced with other groups, such as iodide, cyanide, isothiocyanate, sulfide, polysulfides, and alkyl thiols. These reactions can be used to prepare a variety of allyl derivatives with different properties.
For example, when allyl chloride reacts with sodium sulfite, it yields sodium allyl sulfonate. Sodium allyl sulfonate is a versatile compound that is used in a variety of applications, including cosmetics, pharmaceuticals, and food additives.
In addition to these common reactions, allyl chloride can also undergo a variety of other reactions, such as carbonylation, the formation of organic polycarbonates, and the formation of allyl ethers.
Allyl chloride is a hazardous compound and should be handled with care. It is a flammable liquid and can be a skin and eye irritant. It is also a suspected carcinogen. If you are working with allyl chloride, it is important to wear protective gear, such as gloves, goggles, and a respirator.
3. Production of Allyl Chloride
3.1. Propene Chlorination Process
The extensive production of allyl chloride occurs by the high-temperature (300 – 600 °C) chlorination process of propene:
CH2=CH–CH3 + Cl2 → CH2=CH–CH2Cl + HCl (ΔH298 = -113 kJ/mol)
During these elevated temperatures, the chlorination process progresses via a free-radical chain mechanism. This mechanism predominantly substitutes the hydrogen atom located in the allyl position with chlorine, thereby yielding allyl chloride.
3.1.1. Secondary Reactions and Byproducts
At temperatures below 200 °C, the reaction of propene with chlorine primarily involves the addition to the double bond, resulting in 1,2-dichloropropane formation. As the temperature exceeds 300 °C, the formation of allyl chloride take place, making 1,2-dichloropropane as a byproduct. Additional chlorination products also form in small quantities:
CH3–CH=CH2 + Cl2 → CH3–CHCl–CH2Cl (ΔH298 = -184 kJ/mol)
ClCH2–CH=CH2 + Cl2 → CH2Cl–CH=CHCl + HCl (ΔH298 = -101 kJ/mol)
CH3–CH=CH2 + Cl2 → CH3–C(Cl)=CH2 + HCl (ΔH298 = -121 kJ/mol)
CH3–CH=CH2 + Cl2 → CH3–CH=CHCl + HCl
3.1.2. Critical Process Parameters
In the industrial-scale chlorination of propene to allyl chloride, the most influential factors are the temperature and the propene-to-chlorine ratio. Pressure and residence time have minimal impact on the yield of allyl chloride.
To prevent the production of 1,2-dichloropropane below 200 °C, the mixing temperature of propene and chlorine must be maintained above 250 – 300 °C. The highest yields of allyl chloride are obtained in industrial reactors operating at a peak temperature of 500 – 510 °C.
If the temperature surpasses this range, spontaneous pyrolysis emerges, leading to soot and high-boiling tars. Under experimental conditions at approximately 600 °C, benzene forms, causing a reduction in allyl chloride yield.
3.1.3. Propene-Chlorine Ratio
An escalation in propene excess results in reduced byproduct formation. However, it amplifies propene processing expenses. Consequently, optimal reaction conditions are shaped by economic factors, including the requirement for dichloride byproducts for use as nematicides. These byproducts can also serve as starting materials for the synthesis of C1- or C2-type solvents.
3.1.4. Reactor Pressure and Residence Time
The reactor’s pressure has minimal impact on product yield and distribution. It’s determined solely by the pressure decline within the propene circulation system. Similarly, residence time exerts a limited influence on the allyl chloride yield.
Chlorine reacts completly within 1 – 3 seconds at high temperatures from 300 to 600 °C and excessive residence time leads to the thermal decomposition of allyl chloride.
3.1.5. Reactor Design and Material Selection
Industrial-scale reactors mainly function adiabatically, although optimal yields could be realized with isothermal operation. Due to the rapid and exothermic nature of the reaction, heat loss through the reactor walls is insignificantly low.
The most straightforward and traditional reactor type is the tube reactor, often equipped with gas dispersion and soot removal features. Numerous other reactor configurations are recognized, each striving to facilitate rapid and thorough mixing of reactants to mitigate the formation of 1,3-dichloropropene.
In highly turbulent flow conditions, propene and chlorine are introduced to the reaction zone at velocities up to 300 m/s. The significance of precise mixing conditions is underscored by a cyclone reactor, wherein similar yields are attained at a molar ratio of 3:1 as compared to a ratio of 5:1 with alternative designs. Several reactors have cooling jackets.
By employing a cascade of reactors with distributed chlorine, chlorine conversion rates of up to 86% can be achieved. Such an arrangement permits elevated preheating temperatures due to higher propene:chlorine ratios in the initial reactor.
3.1.6. Feedstock Preparation
Propene and chlorine purity is very important. Impurities in propene trigger byproduct formation and chlorine loss. Notably, propane encourages the development of chlorine derivatives (1-chloropropane, 2-chloropropane) that are challenging to separate.
For the feedstock, propene of polymer-grade purity, at around 99.5%, is often employed. To guarantee sufficient purity, chlorine is revaporized. However, this introduces trace amounts of inert gases, leading to propene loss upon venting.Both propene and chlorine must be as free of water as possible.
3.1.7. Process Overview
A process similar to that employed by Shell is represented in Figure 1.
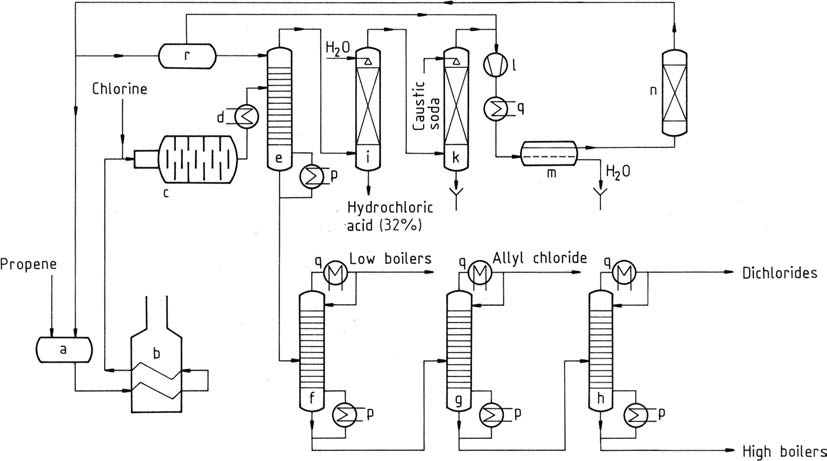
a) Storage vessel for liquid propene; b) Evaporator and superheater for propene; c) Reactor; d) Cooler; e) Prefractionator; f) Light-ends column; g) Allyl chloride purification column; h) Dichloropropene column; i) Hydrogen chloride absorber; k) Gas washer; l) Compressor; m) Decanter for removing water; n) Propene dryer; p) Evaporator; q) Condenser; r) Cold propene storage vessel
Liquid propene is vaporized, followed by preheating to temperatures of 350 – 400 °C (b). This mixture, along with gaseous chlorine, is introduced into the reactor (c) through a mixing jet.
Complete chlorine reaction escalates the temperature to 500 – 510 °C (under optimal conditions). Even in the best circumstances, minimal carbon formation occurs, catalyzing chlorination. A vitreous carbon protective film forms on reactor walls.
This material, comprising highly chlorinated substances and tar, necessitates periodic cleaning every 4 – 8 weeks. Often, parallel reactor chains are employed to maintain partial production during cleaning, or alternating operation of the two chains can be pursued.
The gas stream emerging from the chlorination reactor undergoes precooling (d) and is directed to a prefractionator (e). This prefractionator’s overhead temperature, upheld at around -40 °C, is managed by liquid propene feed. This separates chlorinated hydrocarbons, with the bottom product devoid of propene and hydrogen chloride.
The overhead gaseous mixture is separated through water absorption (i), yielding commercial-grade aqueous hydrogen chloride and propene. Propene is then washed with caustic soda in a scrubber (k) to eliminate traces of hydrogen chloride.
After compression to 1.2 MPa (12 bar), propene is condensed in a condenser (l, q). Liquid propene is dried by adsorption (n) and water is removed (m) then returned to storage (a).
The prefractionator’s bottom product contains 80% allyl chloride, 3% 2-chloro-1-propene, and other low-boiling components, along with 16% dichlorides (primarily 1,2-dichloropropane and cis- and trans-1,3-dichloro-1-propene), and 1% 1,2,3-trichloropropane and other high-boiling fractions. These fractions can be further separated by distillation (f, g, h).
3.1.8. Material Selection for Construction
The choice of materials for allyl chloride synthesis (propene circulation) depends on process specifics, temperature, and pressure. Standard carbon steel can be used where product streams maintain low water content.
If chlorine isn’t preheated, carbon steel can also be employed within the reactor area. Yet, in the reactor region, high-temperature chlorine resistance calls for materials like chromium-nickel steels, nickel, and cupronickel alloys.
Graphite and PTFE coatings are ideal for hydrogen chloride absorption, while rubberized steel suits the caustic soda scrubber. Carbon steel suffices for the entire chlorinated hydrocarbon fractionation plant, although environments with significant water volumes demand expensive materials such as nickel and cupronickel. Dry allyl chloride can be stored in steel vessels without corrosion concerns.
3.2. Other Production Processes
Alternative processes have been proposed for the synthesis of allyl chloride, but none of these methods have been implemented on a commercial scale.
3.2.1. Catalytic Chlorination of Propene
The chlorination of propene in this method uses catalysts containing tellurium, resulting in allyl chloride yields of up to 82%. The primary byproduct is 17% 2-chloropropane (isopropyl chloride). Any unreacted propene and the hydrogen chloride generated during the reaction can be converted into allyl chloride in a subsequent step involving oxychlorination.
3.2.2. Dehydrochlorination of 1,2-Dichloropropane
The dehydrochlorination process of 1,2-dichloropropane leads to a modest yield of only 55% allyl chloride, accompanied by a significant quantity of monochloropropenes. Despite starting with abundant quantities of dichloropropane, which are produced during propene oxide production, this process has no commercial uses.
3.2.3. Oxychlorination
Oxychlorination was developed as an approach to produce allyl chloride by using readily available hydrogen chloride instead of chlorine as the primary feedstock. Various catalyst systems have been proposed, including palladium, vanadium, tellurium, copper, lithium, manganese, as well as their respective chlorides, oxides, and combinations thereof.
While many of these oxychlorination processes initiate from propene, a couple start from propane.
One process, evaluated in a pilot plant by Hoechst, involves the reaction of propene, hydrogen chloride, oxygen, and 2-chloropropane (which can be produced within a subsidiary reactor or introduced externally) in a primary fluid-bed reactor operating at temperatures of 200 – 260 °C and 0.1 MPa (1 bar) gauge pressure (ΔH = -218 kJ/mol).
The catalyst mixture includes tellurium, vanadium pentoxide, phosphoric acid, and a nitrogen compound as a promoter. Part of the catalyst flow is treated with air and nitric acid in a side stream to eliminate coke formation and maintain consistent reactivity.
In a separate reactor, 2-chloropropane is produced from propene, hydrogen chloride, and a ferric chloride solution. The unreacted propene, 2-chloropropane, and hydrogen chloride are separated and recycled.
Allyl chloride yield ranges from 88% to 94% based on propene input. Propene purity isn’t highly critical in this case.
Certain oxychlorination approaches have inherent drawbacks. For instance, catalyst activities decline fast due to the volatility of employed metal salts, necessitating large reactor volumes due to low conversion per pass.
Also, extracting highly diluted allyl chloride from the reaction mixture without excessive propene loss by oxidation poses challenges. A solution to this is usiing manganese dioxide as both a catalyst and oxygen carrier. The main reactions occurring in the primary reactor are as follows:
MnO2 + 4 HCl → MnCl2 + Cl2 + 2 H2O
C3H6 + Cl2 → C3H5Cl + HCl
The catalyst is subsequently reoxidized and activated with oxygen:
MnCl2 + O2 → MnO2 + Cl2
This process achieves allyl chloride yields ranging from 71% to 81% based on propene consumption.
4. Uses of Allyl Chloride
Allyl chloride is an important intermediate in the petrochemical industry. It is used primarily to produce epichlorohydrin, which is used to make epoxy resins. Allyl chloride is also a starting material for the synthesis of various other chemicals, such as glycerol, esters, allyl ethers, and allylamines.
Other compounds that can be made from allyl chloride include allyl isothiocyanate (synthetic mustard oil), allyl sulfonate, allylsilane, and cyclopropane.
Here is a more detailed breakdown of the uses of allyl chloride:
- Epichlorohydrin: Allyl chloride is used to produce epichlorohydrin, which is a versatile compound with a wide range of applications. Epichlorohydrin is used to make epoxy resins, which are used in a variety of products, including adhesives, coatings, and composites. Epichlorohydrin is also used to make glycerol, which is a sweet, colorless liquid that is used in a variety of products, including food, cosmetics, and pharmaceuticals.
- Esterification: Allyl chloride is used in the esterification of phthalic, phosphoric, and carboxylic acids. This process produces esters, which are compounds with a wide range of applications. Esters are used in perfumes, flavorings, and plastics. They are also used as solvents and as intermediates in the production of other chemicals.
- Allyl ethers and allylamines: Allyl chloride is used to produce allyl ethers and allylamines. Allyl ethers are compounds with a characteristic odor that are used in perfumes and flavorings. Allylamines are compounds with a wide range of applications. They are used in pharmaceuticals, agricultural chemicals, and plastics.
- Other compounds: Allyl chloride can also be used to produce other compounds, such as allyl isothiocyanate, allyl sulfonate, allylsilane, and cyclopropane. Allyl isothiocyanate is a compound with a strong odor that is used in plant protection agents and pharmaceutical preparations. Allyl sulfonate is used as an electroplating-bath additive and in the production of carbon fibers. Allylsilane is used for the production of additives for the rubber industry. Cyclopropane is an anesthetic that is used in surgery.
5. Handling and environmental protection of allyl chloride
Allyl chloride is a highly reactive, toxic, and flammable substance. It is therefore subject to strict regulations in many countries regarding its emissions into the atmosphere. Allyl chloride should be handled in closed systems in order to comply with these regulations.
Gaseous mixtures containing allyl chloride or byproducts derived from the production process need to be purified via condensation, absorption, adsorption, or combustion before venting. An effective technique for transferring allyl chloride from one container to another is the compensation technique.
Contamination of soil, groundwater, waterways, or wastewater must be avoided at all costs. Any potential contamination must be reported immediately to the authorities.
Disposal of allyl chloride-containing wastes poses no major hazard when specialized incinerators like those used for solvent disposal are employed. The combustion gases from these incinerators must be treated in absorbers or scrubbers to remove the hydrogen chloride that is formed.
Due to its high volatility and low flash point, plants for the production and processing of allyl chloride must comply with strict fire and explosion safety regulations.
Combustion of chlorinated hydrocarbons produces hydrogen chloride, so firefighters who respond to fires involving allyl chloride must be equipped with protective suits and breathing apparatus. Inadequate air supply during combustion could result in carbon monoxide formation.
Allyl chloride is highly reactive and can react vigorously, exothermically, and even explosively with other substances. This is particularly true of alkali and alkaline-earth metals, but also of aluminum and zinc and strong oxidizing agents, such as concentrated sulfuric acid.
The anhydrous halides (e.g., chlorides) of the metals mentioned above also react vigorously with allyl chloride. Careful consideration must be given to the safety aspects of these exothermic reactions in plants that produce or process allyl chloride.
In 1996, the European Union classified allyl chloride as “dangerous for the environment” and “very toxic to aquatic organisms.”
6. Toxicology and Occupational Health
In laboratory studies involving rats and rabbits, the LD50 values indicate that the oral consumption of 460 mg/kg of allyl chloride proved lethal for rats, while the percutaneous application of 3.7 mg/kg led to mortality in rabbits. Additionally, the inhalation of allyl chloride at a concentration of 11 mg/L for 2 hours resulted in a lethal outcome for rats.
Allyl chloride is a toxic chemical that can be absorbed by inhalation, ingestion, and through the skin.
It is a strong irritant of the skin and mucous membranes, and can cause symptoms such as eye irritation, coughing, shortness of breath, and numbness.
In high concentrations, allyl chloride can cause pulmonary edema (fluid in the lungs), heart damage, liver damage, and kidney damage.
It is also suspected to be a carcinogen and a mutagen.
The threshold limit value (TLV) for allyl chloride is 1 ppm (8 h time weighted average). This means that the average concentration of allyl chloride in the air should not exceed 1 ppm for an 8-hour workday.
The short-term exposure limit for allyl chloride is 2 ppm. This means that the concentration of allyl chloride in the air should not exceed 2 ppm for a period of 15 minutes.
Allyl chloride has a pungent odor, but this odor is not a reliable warning signal. The odor perception threshold for allyl chloride is 3 to 6 ppm, which is considerably above the permissible working concentrations.
There is limited research on the effects of allyl chloride on human health. However, the available evidence suggests that it is a toxic chemical with the potential to cause a variety of health problems, including cancer and reproductive harm.
It is important to note that the information in the text is just a summary of the known toxicity of allyl chloride. The actual effects of exposure to allyl chloride may vary depending on the individual’s exposure level, duration of exposure, and other factors.
References
- Allyl Compounds; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a01_425
- https://www.solvay.com/sites/g/files/srpend221/files/2021-01/PSS-Allyl-Chloride-Epichlorohydrin.pdf
FAQ
Another name for allyl chloride is 3-chloropropene. This alternative name reflects its molecular structure, where a chlorine atom is attached to the third carbon atom in a propene molecule.
Allyl chloride density is 0.939 g/mL at 25 °C.
Allyl chloride is used primarily to produce epichlorohydrin, which is used to make epoxy resins. Allyl chloride is also a starting material for the synthesis of various other chemicals, such as glycerol, esters, allyl ethers, and allylamines.
Allyl chloride is produced by the high-temperature chlorination of propene, a process known as propene chlorination. This involves substituting a hydrogen atom on the allylic carbon with a chlorine atom. Several other methods, such as catalytic chlorination and oxychlorination, are also employed for its production.
Yes, allyl chloride is toxic and can pose health risks upon inhalation, skin contact, or ingestion. It can cause irritation to the respiratory system, skin, and eyes. Prolonged exposure or high concentrations can lead to more severe health effects. Proper protective measures should be taken when handling allyl chloride.
Allyl chloride has a pungent, garlic-like odor. The odor can be detected at very low concentrations.
Allyl chloride is highly reactive and can undergo various chemical reactions. It can react with a range of substances, including water, alcohols, amines, and other nucleophiles. It is particularly reactive with metals, alkali and alkaline-earth metals, as well as strong oxidizing agents like concentrated sulfuric acid. These reactions often involve substitution or addition reactions at the allylic carbon.


