1,4-Benzoquinone: Production, Reactions and Uses

1,4-Benzoquinone, also commonly known as para-benzoquinone or simply quinone, is an organic compound with the formula C6H4O2. It appears as bright yellow crystals with a pungent odor similar to chlorine or bleach.
1,4-benzoquinone was first synthesized in 1838 by oxidizing quinic acid with manganese dioxide. Over fifty years later, the less stable isomer, 1,2-benzoquinone, was prepared by oxidizing catechol with silver ions.
Since then, these vibrant and reactive compounds have been the subject of extensive research, both practical and theoretical.
Table of Contents
1. Physical Properties of 1,4-Benzoquinone
1,4-Benzoquinone is a yellow solid has a molecular weight of 108.10 g/mol. It is soluble in most oxygenated organic solvents, such as ether, ethanol, and acetone, and hot ligroin.
It is slightly soluble in pentane and insoluble in water. It can be crystallized from ethanol or sublimated to produce yellow monoclinic prisms.
| Property | Value |
|---|---|
| Appearance | Yellow monoclinic solid |
| Melting point | 113 °C |
| Density | 1.318 g/cm3 |
| Vapor pressure at 20 °C | 12 Pa |
| Optical rotation | +11.5° (c=1 in ethanol) |
| Refractive index at 20 °C | 1.563 |
2. Chemical Reactions of 1,4-Benzoquinone
Quinones are important oxidants in nature and in synthetic chemistry. They have a versatile reactivity due to their cross-conjugated system of two α,β-unsaturated carbonyl groups.
Michael addition is a common reaction of 1,4-benzoquinone, but other radical and electrophilic reactions are also important. Para-benzoquinone is also an important dienophile for Diels-Alder reactions.
When additions fail, substitution reactions are often used. 1,4-Benzoquinone is also involved in photochemical processes.
The addition of arylsulfinic acids to 1,4-benzoquinone has been studied in detail. The product distribution of this reaction can be strongly influenced by changes in acidity and solvent. Ionic liquids show great promise as green solvents for this chemistry.

The tripeptide glutathione adds to 1,4-benzoquinone to form a thioether. This thioether then undergoes cross-oxidation and further addition to give a mixture of the three possible isomeric products.

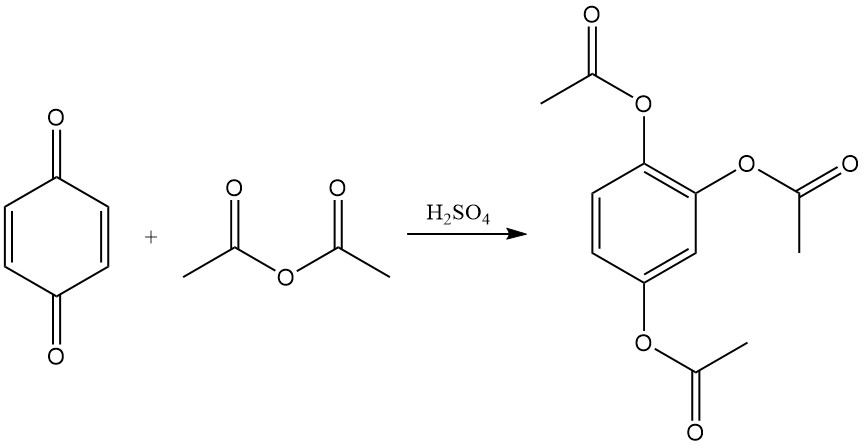
p-Benzoquinone reacts with acetic anhydride and sulfuric acid to produce triacetylhydroxyquinol. This reaction is called the Thiele reaction or Thiele-Winter reaction, named after Johannes Thiele, who first described it in 1898, and Ernst Winter, who further described its reaction mechanism in 1900.
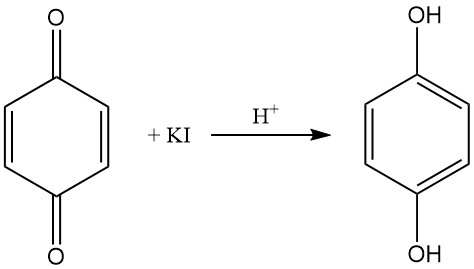
An acidic potassium iodide solution reduces 1,4-benzoquinone in solution to hydroquinone, which can be reoxidized to p-benzoquinone with silver nitrate solution.
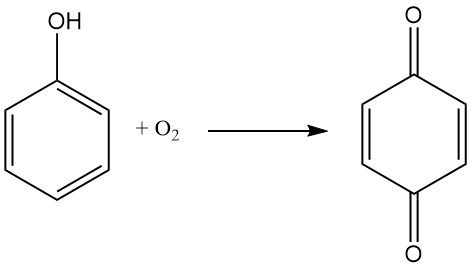
Due to its ability to act as an oxidizer, 1,4-benzoquinone can be used in Wacker-Tsuji oxidation methods, where a palladium salt catalyzes the conversion of an alkene to a ketone. This reaction is typically carried out with pressurized oxygen as the oxidizer, but para-benzoquinone is sometimes preferred. It is also used as a reagent in some variants of Wacker oxidations.
The Meerwein arylation reaction has been reviewed and the importance of the semiquinone radical ion as an intermediate has been established. Radical arylations have been used in the synthesis of natural products.

3. Production of 1,4-Benzoquinone
Quinones, which are considered fine organic chemicals, are usually made using standard oxidation methods. Quinones with high oxidation potentials, such as chloranil (742 mV) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (1000 mV), are often preferred as reagents.
Although many ways to make 1,4-benzoquinone have been proposed, the commercial route usually involves oxidizing aniline or phenol. To obtain a high-yield and high-purity product, steam distillation method is used.
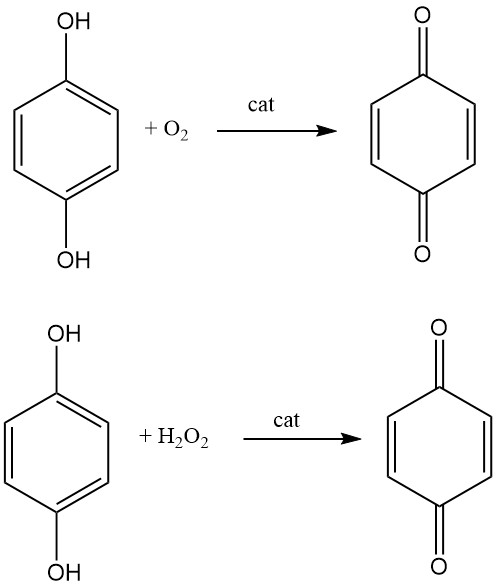
1,4-benzoquinone is produced industrially by the oxidation of hydroquinone with air in the presence of a catalyst such as vanadium pentoxide. The hydroquinone is dissolved in water and heated to a temperature of 70-80 °C. Air is then bubbled through the solution, and the 1,4-benzoquinone is formed as a precipitate. The precipitate is then filtered off and dried.
Glucose is also a possible raw material for making para-benzoquinone.
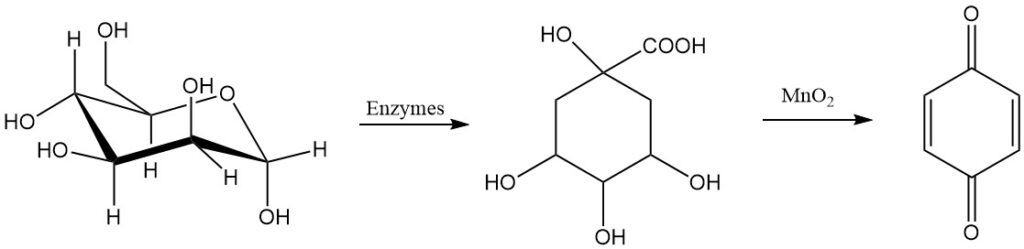
Another process for making para-benzoquinone is the oxidation of hydroquinone with hydrogen peroxide in a liquid phase using iodine, hydrogen iodide, or a metal iodine compound as a catalyst.
The catalysts are preferably soluble in water, such as sodium iodide, potassium iodide, lithium iodide, zinc iodide, magnesium iodide, and alkali metal iodates. 0.8-2.0 mols more hydrogen peroxide is used than hydroquinone.
Hydroquinone is added in isopropyl alcohol at room temperature. Iodine, hydrogen iodide, or a metal iodine compound is then added at 25 °C., followed by the dropwise addition of a 35% hydrogen peroxide solution. The temperature is then raised to 45-50 °C and maintained for several hours to complete the reaction.
Since the reaction product has low solubility in the reaction medium, it can be easily collected by filtering at room temperature to obtain yellow crystals of high-quality, high-purity p-benzoquinone.
The solvent used in the reaction can be recovered by distillation and reused in the reaction, even if it contains water.
1,4-benzoquinone can also be synthesized in the laboratory by the oxidation of hydroquinone with potassium dichromate in sulfuric acid. The hydroquinone is dissolved in water and cooled to 0 °C. Potassium dichromate solution is then slowly added to the hydroquinone solution, with stirring. The 1,4-benzoquinone is formed as a precipitate, which is then filtered off and dried.
4. Uses of 1,4-Benzoquinone
1,4-benzoquinone is a versatile chemical with a wide range of applications. It is used as a polymerization inhibitor, amino acid analysis reagent, and additive in adhesive mixtures.
p-benzoquinone is mainly used as a precursor to hydroquinone, which is used in photography and rubber manufacture as a reducing agent and antioxidant.
It is also an essential tool in research laboratories, particularly for oxidative syntheses, analytical methodologies, and bactericidal agents.
Hydroquinone, a common derivative of 1,4-benzoquinone, is important in the photographic, dye, and leather industries.
1,4-Benzoquinone is used in the production of a variety of dyes, including azo dyes, vat dyes, and sulfur dyes.
p-Benzoquinone is used in the production of a variety of pharmaceuticals, including antibiotics, antitumor drugs, and anticonvulsants. Benzoquinonium is a Skeletal muscle relaxant, ganglion blocking agent that is made from benzoquinone
It is also used in a variety of other applications, including photography, wood preservation, and water treatment.
5. Toxicology of 1,4-Benzoquinone
- 1,4-Benzoquinone and its low-molecular-weight derivatives are hazardous chemicals with a high vapor pressure and penetrating odor.
- Exposure to 1,4-benzoquinone vapor or benzoquinone formed from hydroquinone dust in moist air can cause eye injuries that can lead to blindness.
- The odor of 1,4-benzoquinone is detectable at concentrations of 0.1-0.15 ppm and becomes irritating at 0.5 ppm.
- The occupational exposure limit for benzoquinone is 0.1 ppm (MAK) and 0.4 mg/m3 (TLV-TWA), with short-term exposure limits of 0.3 ppm and 7 mg/m3 (STEL).
- Russia has set a lower threshold limit of 0.01 ppm.
- 1,4-Benzoquinone can cause severe local damage to the skin and mucous membranes, so it must be handled with care.
- Limited carcinogenicity studies on 1,4-benzoquinone in mice and rats have not produced definitive results, so it is classified as a questionable carcinogen.
References
- Benzoquinone; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a03_571.pub2
- Process of preparing p-benzoquinone. – https://patents.google.com/patent/US3859317
- Method for preparing a benzoquinone. – https://data.epo.org/publication-server/document?iDocId=174856&iFormat=2
- Process for the preparation of p-benzoquinone. – https://patents.google.com/patent/US4973720A/en
- 1,4-Benzoquinone, BQ. – https://www.organic-chemistry.org/chemicals/oxidations/bq-1,4-benzoquinone.shtm
- Reactions and applications of 1,4-Benzoquinone. – https://www.chemicalbook.com/article/reactions-and-applications-of-1-4-benzoquinone.htm
