Salicylic Acid: Properties, Production, Uses and Derivatives
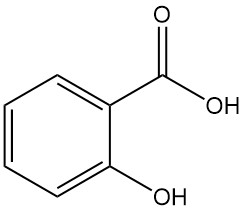
Salicylic acid, also known as 2-hydroxybenzoic acid or o-hydroxybenzoic acid, is an aromatic compound with the formula C7H6O3. It is a colorless, odorless, crystalline solid that is found naturally in many plants, primarily as esters.
Evidence of its medicinal use dates back to ancient Greece. Around 2400 years ago, Hippocrates prescribed willow bark decoctions to treat fever and pain. This therapeutic effect can be attributed to salicylic acid, the active ingredient in willow bark.
The biosynthesis of salicylic acid in plants occurs through the deamination of phenylalanine to trans-cinnamic acid, which is further hydrolyzed and oxidized at the beta-carbon to yield salicylic acid.
Salicylate esters are abundant in various plant genera, including Salix (willow), Spiraea (meadowsweet), and Gaultheria (wintergreen). For example, methyl salicylate, a derivative of salicylic acid, is present in high concentrations as a glucoside in birch bark, certain Spiraea species, and wintergreen leaves.
Salicylic acid was first isolated in 1838 by R. Piria by treating salicylaldehyde (derived from meadowsweet) with potassium hydroxide. Six years later, C. Cahours, a French chemist, obtained salicylic acid by hydrolyzing methyl salicylate.
The production of salicylic acid was achieved in 1874 by H. Kolbe, a German chemist, by carboxylation of sodium phenoxide, a process still employed today.
Salicylic acid and its derivatives are important in various industries. They serve primarily as precursors for the synthesis of pharmaceuticals, dyes, agrochemicals, and perfumery products.
Table of Contents
1. Physical Properties of Salicylic Acid
Salicylic acid is an aromatic o-hydroxy carboxylic acid that exhibits intramolecular hydrogen bonding. This, in contrast to its meta and para isomers, enhances its steam volatility and sublimation tendency. Additionally,the acidity of salicylic acid is significantly higher compared to 3-hydroxybenzoic acid (meta isomer) and 4-hydroxybenzoic acid (para isomer).
The important physical properties of salicylic acid are listed in Table 1.
| Property | Value |
|---|---|
| CAS number | 69-72-7 |
| Formula | C7H6O3 |
| Molar Mass | 138.12 g/mol |
| Crystal Structure | Colorless needles (water) or monoclinic prisms (ethanol) |
| Melting Point | 159 °C |
| Sublimation | Begins at 76 °C |
| Flash Point (Closed Cup) | 157 °C |
| Heat of Sublimation | 81.8 kJ/mol |
| Density | 1.443 |
| Dissociation Constants | K₁ = 1.05 × 10-3 K₂ = 4.0 × 10-14 (19 °C) |
| Vapor Pressure | 1.66 mbar (110 °C) 19.3 mbar (150 °C) |
| Solubility | |
| Solvent | Solubility |
| Methanol | 38.46 g/100 g (21 °C) |
| Ethanol | 34.87 g/100 g (21 °C) |
| Chloroform | 1.55 g/100 g (30 °C) |
| Benzene | 1.00 g/100 g (30 °C) |
| Diethyl Ether | 23.4 g/100 mL (17 °C) |
| Acetone | 31.3 g/100 mL (23 °C) |
| Ammonia | Extremely soluble |
| Liquid Sulfur Dioxide | Insoluble |
The solubility of salicylic acid in water depends on temperature, as shown in Table 2.
| Temperature (°C) | Solubility (g/L) |
|---|---|
| 0 | 0.8 |
| 10 | 1.2 |
| 20 | 1.8 |
| 40 | 3.7 |
| 60 | 8.2 |
| 80 | 20.5 |
2. Chemical Reactions of Salicylic Acid
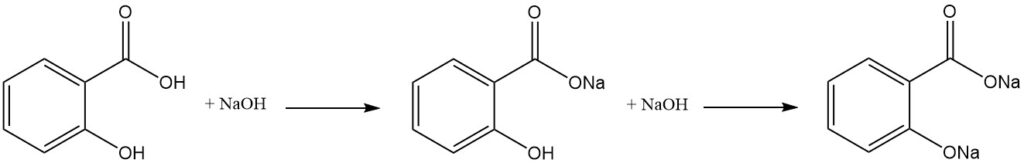
Salicylic acid is a difunctional molecule that has properties characteristic of both phenols and aromatic carboxylic acids. Due to the difference in acidity between the hydroxyl and carboxylic groups, one equivalent of a strong base selectively neutralizes the carboxyl group.
The formation of the dialkali salt requires an excess of base, while subsequent exposure to carbon dioxide results in the reformation of the free hydroxyl group.
Chelation with certain metal ions, like Fe(III), forms violet-colored complexes. This property allows salicylic acid to be used as an indicator in EDTA titrations for Fe(III).
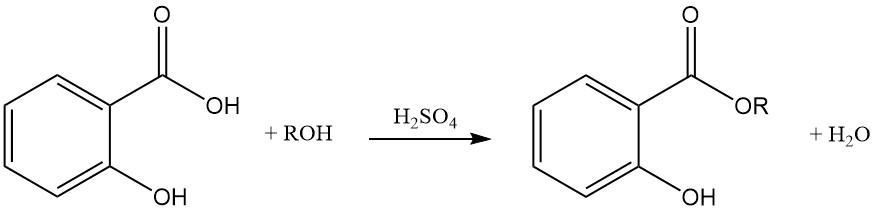
The reaction of salicylic acid with alcohols using strong acids as catalysts produces esters with minimal ether formation as a side product. Esterification can also be achieved with acyl halides or acid anhydrides.
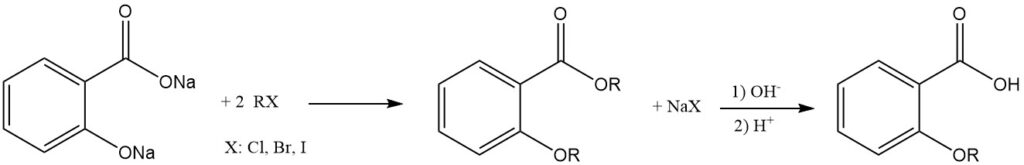
Dialkali salicylate reacts with alkyl halides to yield combined ether esters. Treatment of these products with a base hydrolyzes them to the corresponding alkoxybenzoic acids.
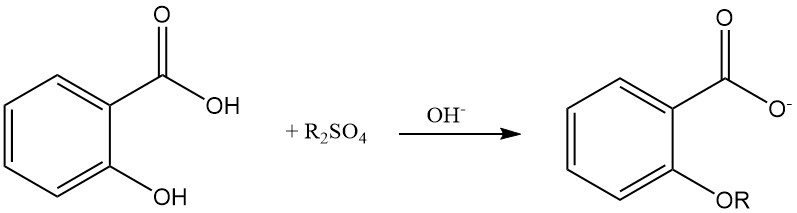
Etherification of the phenolic hydroxyl group of salicylic acid is possible using dialkyl sulfate in an alkaline aqueous solution.
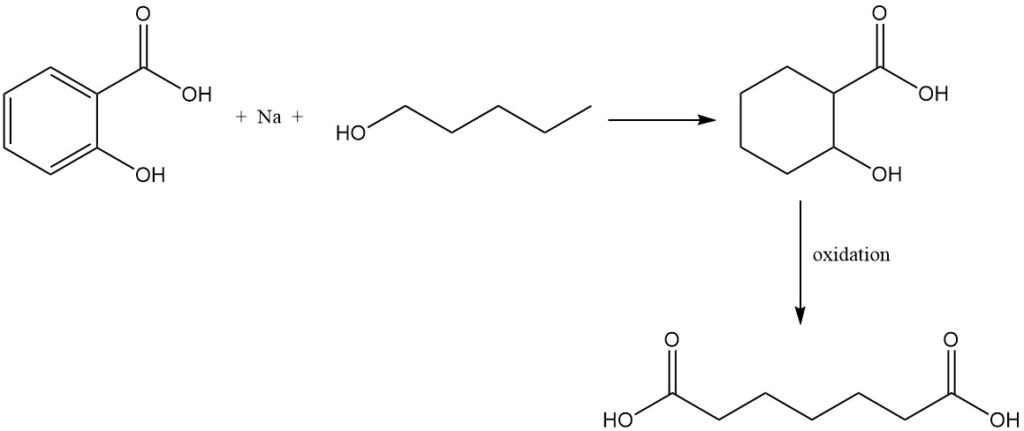
Reduction of salicylic acid with sodium and amyl alcohol forms tetrahydrosalicylic acid, which, upon oxidation, yields pimelic acid.
Catalytic hydrogenation of salicylic acid esters using Raney nickel produces esters of cis-trans-2-hydroxycyclohexane carboxylic acid.
Electrophilic substitution reactions favor the less-hindered 5-position of the aromatic ring. This regioselectivity allows for the direct synthesis of either 5-substituted or 3,5-disubstituted salicylic acid derivatives.
Examples of electrophilic substitution of salicylic acid include nitration, sulfonation, halogenation, alkylation, acylation, and coupling with diazonium salts, which typically produces 5-substituted products.
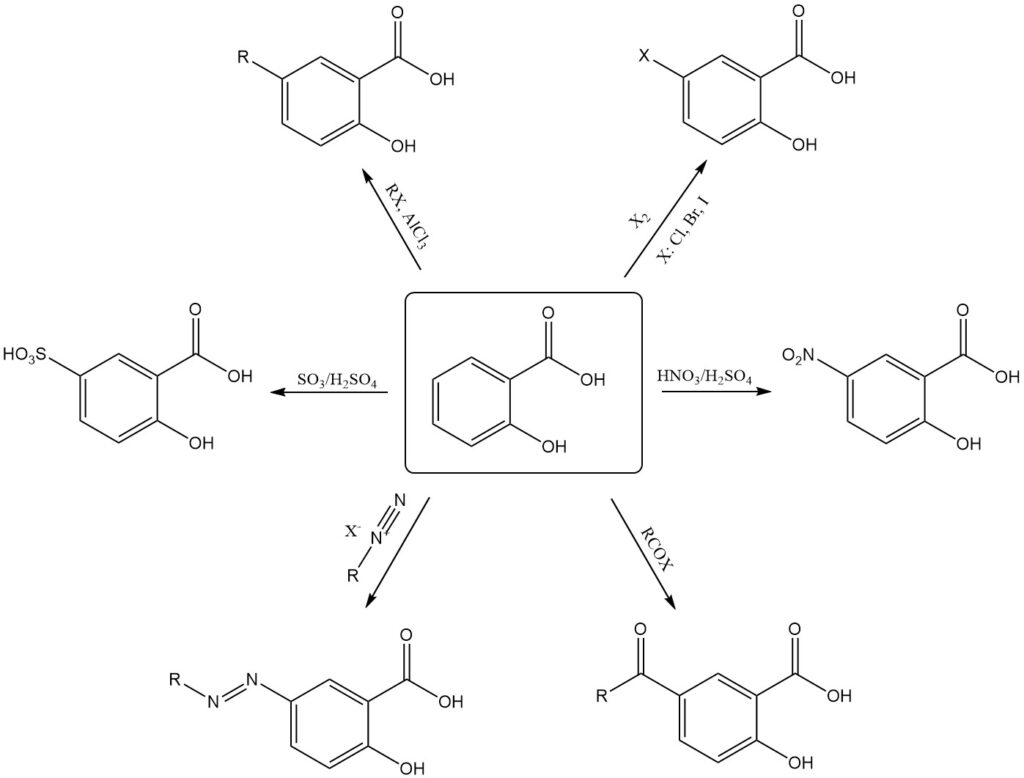
Substituted salicylic derivatives at the 3-position require indirect methods such as the substitution of sulfosalicylic acid at the 5-position, followed by the elimination of the sulfonic acid group. However, harsher reaction conditions may lead to decarboxylation.
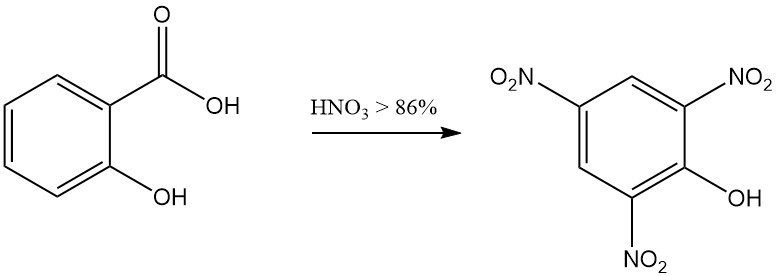
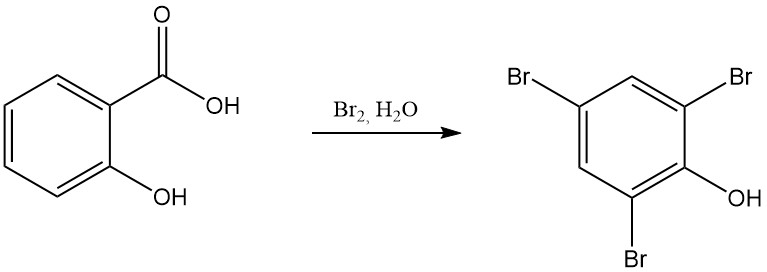
For example, the reaction of salicylic acid with fuming nitric acid results in the formation of 2,4,6-trinitrophenol (picric acid), and the reaction with bromine in the presence of water yields tribromophenol.
The thermal decomposition of salicylic acid at or above its melting point gives phenol and carbon dioxide. Under a carbon dioxide atmosphere at 230 °C, the primary product is phenyl salicylate. At higher temperatures (250 °C), xanthone is formed alongside phenol.
3. Production of Salicylic Acid
3.1. Production of Salicylic Acid by Kolbe-Schmitt Synthesis
Salicylic acid is commercially prepared via the Kolbe-Schmitt reaction. This process involves reacting dry sodium phenoxide with carbon dioxide under pressure (5 bar) and at temperatures ranging from 150 to 160 °C.
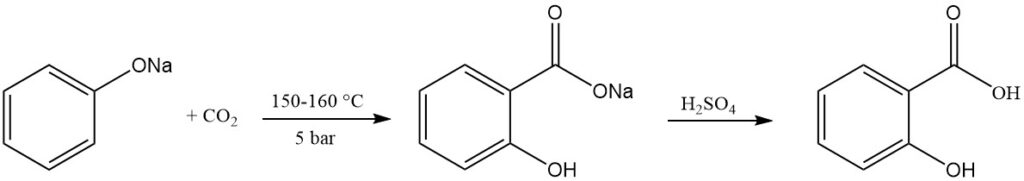
In 1884, Schmitt found that the use of pressure improved the yield to around 90% compared to the original reaction by Kolbe in 1874, which yielded only around 50% at normal pressure. Without pressure, disodium salicylate and phenol form in equal amounts.
The exact mechanism of the Kolbe-Schmitt reaction remains a topic of discussion. Many suggestions have been proposed in the literature, such as:
- 4-Hydroxyisophthalic acid may act as an intermediate, releasing CO2 or undergoing carboxylation of phenolate to form salicylic acid.
- Complex formation between phenol, CO2, and an alkali metal might facilitate electrophilic substitution at the phenoxide.
Temperature, the type of alkali metal used, and CO2 pressure all significantly impact the reactivity and selectivity of the phenoxide anion.
A simplified overview of industrial salicylic acid synthesis is presented in Figure 1.
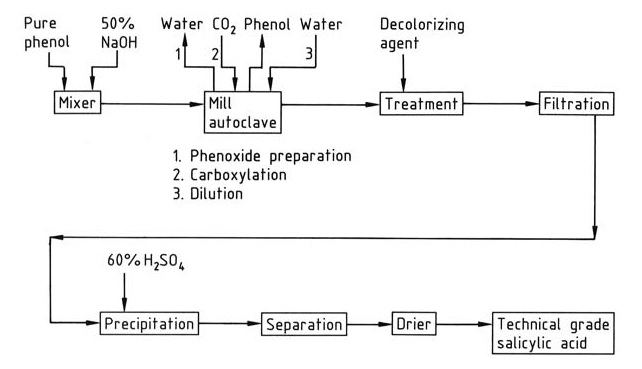
Carboxylation occurs in autoclaves equipped with stirrers or milling devices. The reaction mixture must be finely ground and rigorously free of water to ensure optimal yield. Water reduces yield by converting phenoxide to phenol and promoting the formation of carbonate.
A slight molar excess (1-2%) of sodium hydroxide is used to prepare sodium phenoxide. Larger amounts of caustic soda lead to water formation. The anhydrous sodium phenoxide is prepared by the evaporation of an aqueous sodium phenoxide solution within the autoclave mixer itself, by applying a vacuum after reaching normal pressure, or by using dedicated drying equipment.
The carbon dioxide used in the reaction must have a minimal oxygen content (<0.1%) to prevent discoloration and tar formation.
The exothermic carboxylation reaction converts sodium phenoxide into salicylic acid. The resulting crude sodium salicylate is dissolved in water, treated with a decolorizing agent (e.g., activated charcoal, aluminum, or zinc powder), and then precipitated with sulfuric acid.
Unreacted phenol is recovered from the crude sodium salicylate mixture by distillation.
As an alternative, the continuous process uses a solution of anhydrous phenoxide in a suitable solvent, such as phenol itself, higher alcohols, dialkyl ketones, or nitrobenzene.
3.2. Production of Salicylic Acid by Other Processes
Other alternative methods for producing salicylic acid have been explored:
- Air oxidation of o-cresolate at 230 °C with a copper-based catalyst or copper benzoate at 175-215 °C.
- Heating alkaline copper benzoate directly can yield salicylate.
- The hydrolysis of 2-benzoyloxybenzoic acid, an intermediate in the Dow process for phenol production, leads to the formation of salicylic acid.
- Fermentation of polycyclic aromatic compounds like naphthalene by certain microorganisms can produce salicylic acid.
4. Uses of Salicylic Acid
Salicylic acid is primarily used as a precursor for acetylsalicylic acid (aspirin), the most widely dispensed pharmaceutical globally. Additionally, salicylic acid esters, amides, and salts are starting materials for other pharmaceutical products.
Technical-grade salicylic acid is used as an intermediate in the production of agrochemicals, dyes and colorants, rubber products, and phenolic resins.
Salicylic acid itself possesses therapeutic effects in treating rheumatic conditions. It is typically administered as the highly soluble sodium salt form for this purpose.
Due to its keratolytic properties (ability to break down keratin), salicylic acid is used in various skin care products for cleaning and removing scales.
Salicylic acid is a bactericide used in some disinfectants or preservatives. However, its use in food products is prohibited.
Recently, it has been used as a regulator of plant growth and flowering and against various abiotic and biotic stresses.
Acetylsalicylic acid accounts for roughly 55% of the total salicylic acid market, followed by esters and salts (18%), resins (10%), and dyes and colorants (10%).
The introduction of newer analgesics that compete directly with aspirin has impacted the salicylic acid market, leading to a decline in aspirin consumption. However, the potential discovery of new applications for salicylic acid could stabilize the market in the future.
5. Salicylic Acid Derivatives
Salicylic acid readily forms salts upon reacting with metal carbonates. To prevent discoloration, it’s recommended to keep aqueous salt solutions slightly acidic. Salts are obtained as solids by concentrating their solutions.
5.1. Sodium Salicylate
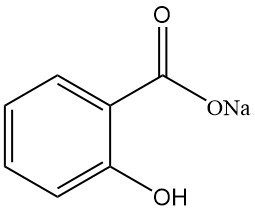
- Formula: NaC7H5O3
- CAS Number: 54-21-7
- Molar Mass: 160.11 g/mol
- Appearance: White, odorless, shiny crystalline flakes
- Solubility:
- Water: 125 g/100 mL (25 °C)
- Ethanol: 17 g/100 mL (15 °C)
Technical-grade sodium salicylate is produced by the evaporation of a sodium salicylate solution, and pharmaceutical-grade sodium salicylate is prepared by the double crystallization of sodium salicylate hexahydrate from a 45% aqueous solution at 10 °C.
It is used as an analgesic, antipyretic, and antineuralgic agent.
Numerous other salicylate salts exist, including those of ammonium, magnesium, calcium, and aluminum, as well as morpholine salicylate. Many of these salts are available under various trade names.
5.2. Methyl Salicylate
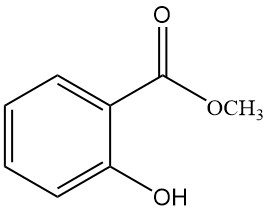
- Formula: C8H8O3
- CAS Number: 119-36-8
- Molar Mass: 152.15 g/mol
- Appearance: Colorless, oily liquid with a characteristic odor
- Melting Point: -9 °C
- Boiling Point: 222 °C
- Density: 1.184 g/mL
Methyl salicylate is produced by heating a mixture of salicylic acid and methanol with sulfuric acid.
It is used in the treatment of neuralgia and rheumatism, stimulating capillary blood circulation, as an insecticide, sunscreen, as fragrance and synthetic intermediate.
5.3. Benzyl Salicylate
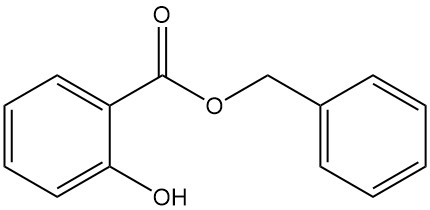
- Formula: C14H12O3
- CAS Number: 118-58-1
- Molar Mass: 228.25 g/mol
- Appearance: Clear liquid or colorless to opaque crystalline mass with a characteristic odor
- Melting Point: 24 °C
- Boiling Point: 318 °C
- Density: 1.180 g/mL (at 15 °C)
Benzyl salicylate is produced by the reaction between sodium salicylate and benzyl chloride or by the transesterification of methyl salicylate with benzyl alcohol.
It is found in ylang-ylang and carnation oils and is used as an additive in soaps, detergents, and perfumes.
5.4. Isoamyl Salicylate
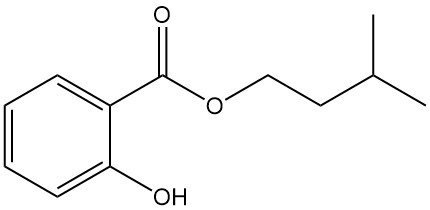
- Formula: C12H16O3
- CAS Number: 87-20-7
- Molar Mass: 208.26 g/mol
- Appearance: Colorless liquid with the fragrance of orchids
- Boiling Point: 270 °C
- Density: 1.050 g/mL (at 20 °C)
Isoamyl salicylate is used as a fragrance stabilizer in perfumery and as a topical antirheumatic agent.
5.5. Phenyl Salicylate
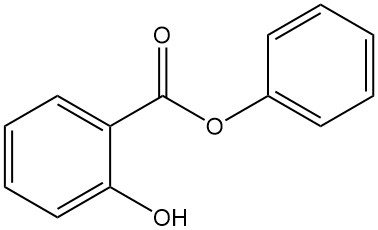
- Formula: C13H10O3
- CAS Number: 118-55-8
- Molar Mass: 241.22 g/mol
- Appearance: Colorless, crystalline powder
- Melting Point: 43 °C
- Boiling Point: 172 °C (at 16 mbar)
Phenyl salicylate is prepared by the reaction between salicylic acid and phenol with sulfuric acid or by the transesterification of methyl salicylate with sodium phenoxide.
It is used as an antiseptic, a preservative, in sunscreen, as a general photoprotective agent for synthetic products, and as an emollient.
5.6. Acetylsalicylic Acid (Aspirin)
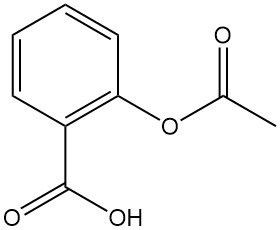
Acetylsalicylic acid, also widely known by the brand name Aspirin, is a key derivative of salicylic acid. Here’s a breakdown of its properties and applications:
Physical Properties:
- Formula: C9H8O4
- Molar Mass: 180.15 g/mol
- Appearance: Colorless needles or crystalline powder (depending on crystallization solvent)
- Melting Point: 143–144 °C
- Solubility: Low in water (0.25 g/100 mL at 15 °C), but more soluble in ethanol (20 g/100 mL at 25 °C)
- Dissociation Constant (K): 2.8 x 10-4 (at 25 °C)
6. Toxicology of Salicylic Acid
Salicylic acid and its derivatives are readily absorbed through the skin and mucous membranes. Ester forms of salicylic acid undergo hydrolysis in the body due to the action of enzymes called esterases.
The elimination pathway for salicylic acid depends on urine pH:
- Acidic Urine: Salicylic acid gets oxidized to gentisic acid.
- Alkaline Urine: Salicylic acid is eliminated by the kidneys as salicyluretic acid or glucuronide salicylate.
Since absorption can be faster than elimination, salicylic acid accumulation in the body can occur under certain conditions.
Biological Effects
Salicylic acid has keratolytic activity, meaning it promotes the shedding of the outermost layer of skin. It can also irritate skin tissues, particularly in the stomach, where it affects mucus-producing cells. Long-term use of salicylates can slow blood clotting by reducing platelet aggregation.
Salicylic acid and its derivatives inhibit prostaglandin synthesis, contributing to their anti-inflammatory properties. These compounds exhibit analgesic and antipyretic effects.
Salicylic acid and its derivatives can act as fungicides and bacteriostatic agents.
Skin and lung hypersensitivity reactions to salicylates are known, although not always true allergies. Reduced prostaglandin synthesis may be involved. Cross-sensitization between different salicylate forms, like methyl salicylate and aspirin, has been observed.
Toxicity
Single doses of salicylic acid exceeding 10 grams can be fatal. The primary concern is disruption of the body’s acid-base balance. Symptoms include delirium, tremors, respiratory issues, sweating, dehydration, fever, and coma.
Less severe intoxication presents with hyperventilation, ringing in the ears, nausea, vomiting, vision or hearing problems, dizziness, and nervous system disturbances.
Chronic use can lead to digestive problems, stomach and intestinal pain, and sometimes serious, hidden bleeding. Salicylate-induced anemia, due to iron deficiency from hidden bleeding, can also occur. In elderly patients, chronic intoxication may manifest as confusion and agitation.
Despite potential tissue damage, chronic administration of pure salicylic acid has not been linked to liver or kidney problems.
Animal studies suggest that high doses of salicylic acid and its derivatives may pose birth defect risks. However, this hasn’t been confirmed in humans, and proper hygiene and working conditions are believed to reduce these concerns.
References
- Salicylic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a23_477
- Analgesics (N02); Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/9783527306732.a02_269.pub4
- Salicylic Acid: A Regulator of Plant Growth and Development
- Salicylic Acid. – https://www.sciencedirect.com/science/article/abs/pii/B9780080552323625759
