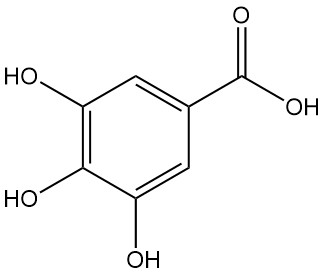
3-hydroxybezoic acid is one of three isomers of hydroxybenzoic acid, the other two being 2-hydroxybenzoic acid and 4-hydroxybenzoic acid with the formula C7H6O3. It is also known as m-hydroxybenzoic acid or m-salicylic acid. It is a white, odorless solid that is soluble in water and ethanol.
Table of Contents
Occurrence of 3-Hydroxybenzoic Acid in plants
3-Hydroxybenzoic acid is a natural product that can be found in various plants, such as:
- Vanilla: The vanilla bean contains about 2% of 3-hydroxybenzoic acid, which contributes to its characteristic flavor and aroma.
- Raspberry: The raspberry fruit contains about 0.2% of 3-hydroxybenzoic acid, which gives it a sour taste and a red color.
- Tea: The tea leaves contain about 0.1% of 3-hydroxybenzoic acid, which acts as an antioxidant and a preservative.
1. Physical Properties of 3-Hydroxybenzoic acid
3-Hydroxybenzoic acid is a non-flammable solid. It is a hygroscopic and a non-volatile solid which is relatively stable, but it can decompose when heated above its melting point. 3-hydroxybenzoic acid is insoluble in non-polar solvents such as hexane and chloroform.
Some of the physical properties of 3-hydroxybenzoic acid are listed in the following table:
| Property | Value |
|---|---|
| Molecular formula | C7H6O3 |
| Molecular weight | 138.12 g/mol |
| Appearance | White needles (when crystallized from water) or rhombic prisms (when crystallized from alcohol) |
| Melting point | 203 °C |
| Boiling point | 297 °C |
| Density | 1.473 g/cm³ at 25°C |
| Dissociation constants | K1 = 8.71 × 10⁻⁵ and K2 = 1.18 × 10⁻¹⁰ at 19°C |
| Solubility | 6.11 g in 100 g of water at 69°C
39.6 g in 100 g of 98% ethanol at 65°C 20.7 g in 100 g of n-butanol at 36.5°C |
2. Chemical Reactions of 3-hydroxybenzoic acid
3-hydroxybenzoic acid is stable at high temperatures, remaining unchanged even at 300 °C. This is a unique property not shared by its counterparts, 2- and 4-hydroxybenzoic acids.
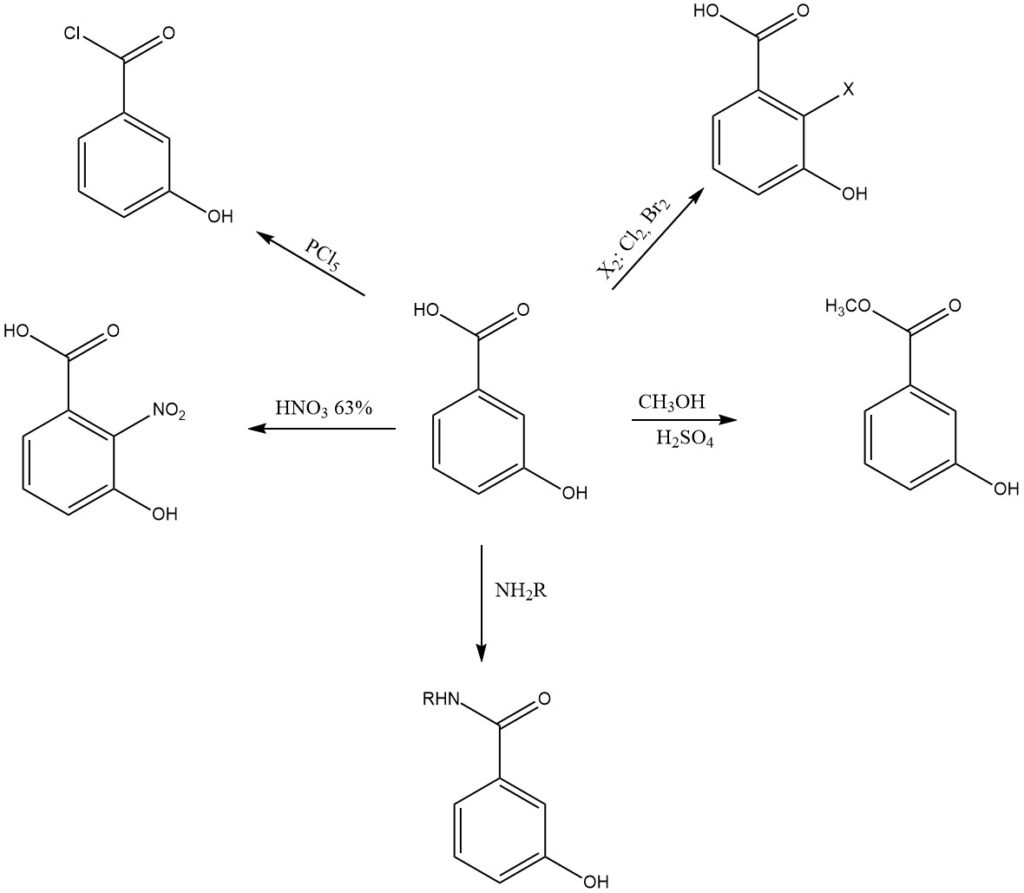
When 3-hydroxybenzoic acid undergoes electrophilic substitution reactions such as nitration, halogenation, or sulfonation, the reactions occur mainly at the ortho or para positions relative to the hydroxyl group.
For example, nitration of 3-hydroxybenzoic acid with 62% aqueous nitric acid produces 2-nitro-3-hydroxybenzoic acid as the main product, along with smaller amounts of 4-nitro- and 6-nitro-3-hydroxybenzoic acids.
Esterification of 3-hydroxybenzoic acid forms a variety of esters, including methyl 3-hydroxybenzoate, ethyl 3-hydroxybenzoate, and propyl 3-hydroxybenzoate. These esters are used as preservatives, fragrances, and flavorings.
3-hydroxybenzoic acid can be amidated to form a variety of amides, including 3-hydroxybenzamide and 3-hydroxy-N-methylbenzamide. These amides are used in a variety of applications, including as pharmaceuticals, dyes, and pigments. It can be reduced to form 3-hydroxybenzyl alcohol.
When reacted with phosphorus pentachloride it forms 3-hydroxybenzoyl chloride. This acyl chloride is used in the synthesis of a variety of esters, amides, and other derivatives. It also reacts with sodium hydroxide to form sodium 3-hydroxybenzoate.
3. Production of 3-hydroxybenzoic acid
3-hydroxybenzoic acid can be produced using several methods:
3.1. Alkali fusion of sodium 3-sulfobenzoate
One of the most common methods of production of 3-hydroxybenzoic acid is the alkali fusion of sodium 3-sulfobenzoate is melted with alkali at 210-220 °C. The resulting crude 3-hydroxybenzoic acid is precipitated after acidification and purified by recrystallization from water with activated carbon. This yields 3-hydroxybenzoic acid with a purity of about 90%.

3.2. Reduction of 3-nitrobenzoic acid esters
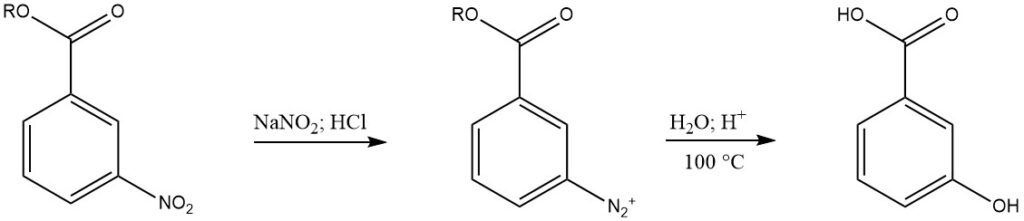
3-Nitrobenzoic acid esters are catalytically hydrogenated to aminobenzoic acid esters, which are then diazotized. The diazonium product is then treated 3-nitrobenzoic acid with water and sulfuric acid at 100 °C for several hours, followed by neutralization and filtration. This methode produces 3-hydroxybenzoic acid with a purity of about 95%.
3.3. Oxidation of 3-methylphenol or 3-hydroxybenzaldehyde
3-hydroxybenzaldehyde is oxidized by bubbling air through a hot aqueous sodium hydroxide suspension to produce 3-hydroxybenzoic acid.
Other method is the oxidation 3-methylphenol with potassium permanganate in alkaline solution, followed by acidification and recrystallization which yields 3-hydroxybenzoic acid with a purity of about 85%.

3.4. Biosynthesis
3-hydroxybenzoic acidcan also be produced biosynthetically by the fermentation of microorganisms such as Escherichia coli and Bacillus subtilis. These microorganisms have been genetically engineered to produce 3-hydroxybenzoic acid from glucose, glycerol, or 3-chlorobenzoic acid.
The 3-hydroxybenzoic acid produced by fermentation can then be purified and recovered.
4. Uses of 3-hydroxybenzoic acid
3-hydroxybenzoic acid has many uses in different industries because of its versatile properties:
- Pharmaceuticals and pesticides: 3-hydroxybenzoic acid is a key building block in the synthesis of many pharmaceuticals and pesticides.
- Ophthalmology: 3-hydroxybenzoic acid esters are used to dilate the pupils in ophthalmic procedures and examinations.
- Digestion: Sodium 3-hydroxybenzoate is a cholepoietic agent, which means it helps to produce and flow bile, which is essential for digestion.
- Food preservation: Esters and metal salts of 3-hydroxybenzoic acid have germicidal and preservative properties, making them useful for extending the shelf life of food products.
- Plastics: Ether-esters derived from 3-hydroxybenzoic acid can be used as plasticizers for vinyl and cellulose resins, making them more flexible and workable.
- Cosmetics: Esters of 3-hydroxybenzoic acid are used in cosmetics as preservatives and UV absorbers.
- Personal care products: 3-hydroxybenzoic acid and its derivatives are used in personal care products such as toothpaste, mouthwash, and deodorant.
- 3-hydroxybenzoic acid is used in the textile industry as a dye intermediate.
- Analytical chemistry: 3-hydroxybenzoic acid is used in analytical chemistry as a reagent for detecting metals and other ions.
3-hydroxybenzoic acid is also being investigated for its potential use in the treatment of various diseases, including cancer, Alzheimer’s disease, and Parkinson’s disease.
5. Toxicology of 3-hydroxybenzoic acid
3-hydroxybenzoic acid is a relatively safe chemical.
- LD50 (rat, i.p.) = 3700 mg/kg
- LD50 (mouse, oral) = 2 g/kg
- TDLo (rat, s.c., 11 d pregnant) = 400 mg/kg
- Classification: drug, teratogen
- Harmful if swallowed
- Causes skin irritation
- Causes serious eye irritation
- May cause respiratory irritation
- Toxicity to algae: Growth inhibition EC50 (Scenedesmus quadricauda) > 10 mg/L – 13 days
References
- Hydroxycarboxylic Acids, Aromatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_519
Process for making mu-hydroxybenzoates. – https://patents.google.com/patent/US3094558A/en
- Establishing microbial co‐cultures for 3‐hydroxybenzoic acid biosynthesis on glycerol. – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6999546/