Benzylamine: Properties, Production and Uses
Table of Contents
1. Physical Properties of Benzylamine
| Property | Value |
|---|---|
| Synonyms | α-aminotoluene, phenylmethylamine |
| Chemical formula | C7H9N |
| Molecular weight | 107.16 g/mol |
| Physical appearance | Colorless liquid with a weak amine-like odor |
| Solubility | Miscible with water, alcohol, and diethyl ether |
| Boiling point | 184.5 °C at 101.3 kPa 90 °C at 1.6 kPa |
| Solidification point | < -30 °C |
| Vapor pressure | ca. 60 Pa at 20 °C ca. 130 Pa at 30 °C 520 Pa at 50 °C |
| Heat of vaporization | 49 kJ/mol |
| Heat of combustion | 4058.7 kJ/mol at 101.3 kPa and 20 °C |
| Refractive index at 20 °C | 1,5401 |
| Density at 20 °C | 0.9813 |
| Dielectric constant | 4.6 at 20.6 °C |
| Electrolytic dissociation constant in water | 2.35 × 10-5 at 20 °C |
| Viscosity | 1.78 mPa·s at 21.2 °C 0.295 mPa·s at 178.2 °C |
| Surface tension | 38.82 × 10-5 N/cm at 21.1 °C 31.70 × 10-5 N/cm at 88 °C |
| Autoignition temperature | 390 °C |
| Flash point | 65 °C |
| Explosion limit | 0.9% (lower) to 14% (upper) |
| Dipole moment (20 °C) | 1.18 D (neat), 1.25 D in toluene, 1.29 D in hexane, 1.30 D in cyclohexane, 1.33 D in benzene |
| Dynamic viscosity | 0.01596 g cm-1 s-1 at 25 °C |
| Absorption maximum lmax in water | 255.9 nm (e 249 L mol-1 cm-1) |
| Magnetic susceptibility | −75.3 × 10-6 cm3/mol |
2. Chemical Reactions of Benzylamine
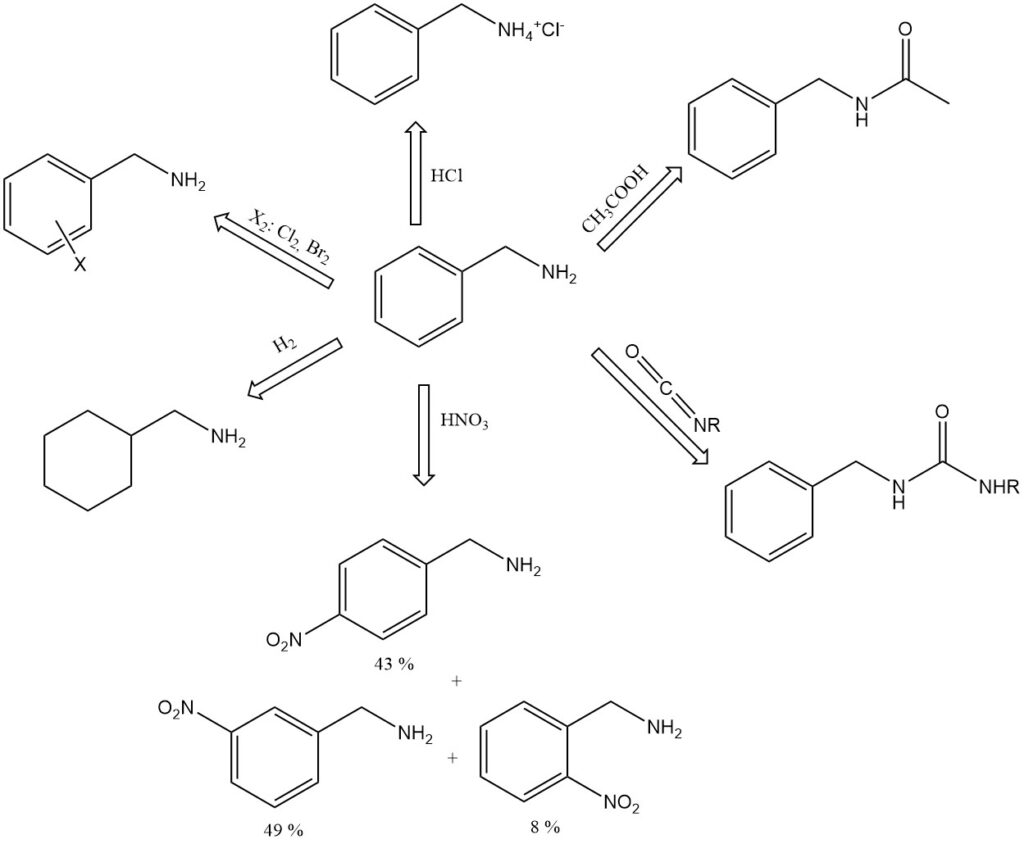
Benzylamine is a stronger base than its toluidine isomers and is strongly alkaline even when diluted with some water (pH 11.6 in 100 g/L water).
It forms addition compounds with phenol (1:3, mp 15.3 °C), p-cresol (1:1, mp −6 °C), and formic acid (1:1, mp 81 °C). Examples of benzylamine salts include the hydrochloride, C6H5CH2NH+3 Cl−, mp 260 °C (decomp.), and the picrate, mp 194 °C.
Benzylamine absorbs carbon dioxide from the air to form a solid carbamic acid salt.
When boiled with glacial acetic acid, benzylamine forms N-acetylbenzylamine.
Benzylamine reacts with isocyanates to give substituted ureas, C6H5CH2NHCONHR.
Nitric acid nitrates benzylamine on the aromatic nucleus to give 8% ortho-, 49% meta-, and 43% para-nitrobenzylamine.
Catalytic hydrogenation of benzylamine gives hexahydrobenzylamine, C6H11CH2NH2.
Benzylamine can undergoe aromatic reactions on the benzene ring such as alkylation, acylation, sulfonation and halogenation.
3. Production of Benzylamine
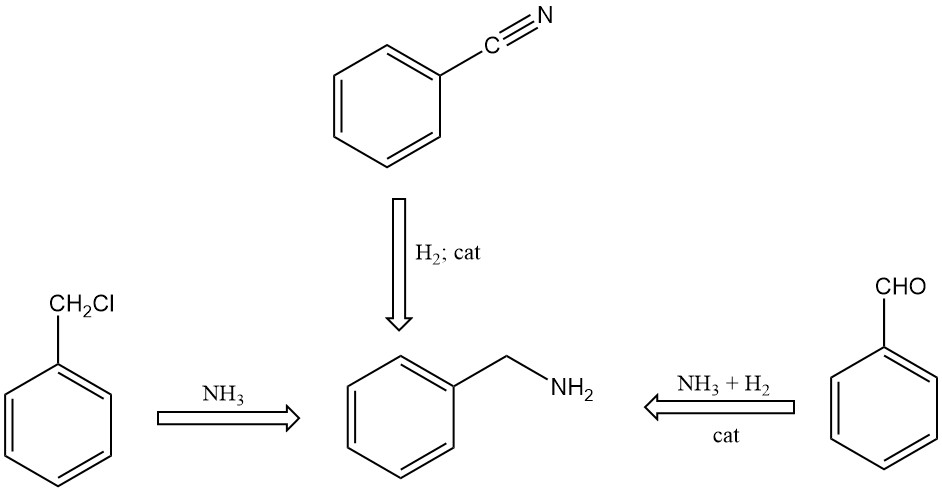
There are several methods for synthesizing benzylamine:
- Reaction of benzyl chloride with ammonia in an aqueous solution
- Catalytic hydrogenation of benzonitrile
- Reaction of benzaldehyde with ammonia in the presence of hydrogen and catalysts, using organic solvents in the reaction mixture
Benzylamine is produced by the benzaldehyde route as follows:
- Combine 8 kg of Raney nickel (equivalent to about 4 kg of 100% Ni), 10 g of glacial acetic acid, 110 kg of ammonia, and a mixture of 250 kg of methanol and 500 kg of benzaldehyde in a 1200-L steel autoclave.
- Hydrogenate the mixture at 100 °C and 15 MPa for 3 to 5 hours, replenishing the hydrogen every 10 minutes or so. After hydrogen consumption ceases, maintain the reaction vessel at the same temperature and pressure for an additional 30 minutes.
- Separate the catalyst from the reaction products using a pressure filter. The filtrate contains approximately 470 kg of benzylamine for a yield of 93%, along with trace amounts of benzyl alcohol, dibenzylamine, Schiff base (N-benzalbenzylamine), methanol, reaction water, and ammonia, for a yield of 93%.
- Vacuum distillation is used to obtain benzylamine of the necessary technical purity.
Benzylamine has also been identified in the leaves and flowers of the plant Reseda media.
4. Uses of Benzylamine
Benzylamine is used in the production of synthetic textiles, paints, and corrosion inhibitors. It is also used as an intermediate in the production of pharmaceuticals and pesticides. In these cases, benzylamine is often used as a protected nitrogen.
Benzylamine is a versatile intermediate and building block in a variety of applications, including coatings and crop protection.
It is used in the industrial manufacture of numerous pharmaceuticals, including alniditan, lacosamide, moxifloxacin, and nebivolol.
Benzylamine is also used to manufacture the military explosive hexanitrohexaazaisowurtzitane (HNIW).
5. Toxicology of Benzylamine
Benzylamine is readily biodegradable, but it is harmful to aquatic life, with a weak effect (German class for hazards in aquatic environment: 1, WGK 1).
Acute toxicity of benzylamine varies among organisms:
- Pseudomonas fluorescens (bacteria): EC0 500 mg
- Scenedesmus quadricauda (algae): 96-h EC10 6 mg
- Daphnia magna: 48-h EC50 60 mg
- Leuciscus idus (fish): 48-h EC0 20 mg
- Pimephals promelas (fish): 96-h EC50 102 mg
Benzylamine can cause skin and eye burns and may lead to sensitization. If exposed to benzylamine, immediately rinse with water and wash the skin with soap. A 5% aqueous solution of acetic acid should be accessible to neutralize contaminated skin. If ingested, dilute by drinking water and rinse mouth. In cases of inhalation risk, use ABEK filters in breathing masks (DIN 3181).
Benzylamine showed a negative result in the Ames test, indicating no mutagenic effect. The acute oral and percutaneous LD50 values for rats are 1130 mg/kg and 1340 mg/kg, respectively. It is classified as Toxicity class 3 in Switzerland.
Rats survived a two-week period after 3 hours of full body inhalation exposure to benzylamine, but 17% of rats died after 5 hours of exposure.
References
- Benzylamine; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_009.pub2
- https://products.basf.com/global/en/ci/benzylamine.html
