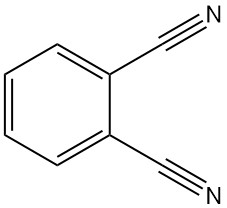
What is Phthalonitrile?
Phthalonitrile, also known as 1,2-dicyanobenzene or 1,2-benzenedicarbonitrile, is an organic compound with the formula C8H4N2. It is a crystalline powder with a faint grayish-yellow color and a faint aromatic odor, similar to benzonitrile.
Phthalonitrile was first discovered in 1896, during the diazotization of 2-aminobenzonitrile.
Table of Contents
1. Physical Properties of Phthalonitrile
Phthalonitrile is a crystalline solid that is not soluble in water (around 1 g/L) but is soluble in organic solvents like ethanol, acetone, nitrobenzene, benzonitrile, and ether.
Phthalonitrile can’t be distillated and undergoes polymerization when heated above its melting point. It is not explosive, and its ignition is difficult, but the dust can explode.
Table 1 presents the important physical properties of phthalonitrile.
| Property | Value |
|---|---|
| CAS Number | []91-15-6] |
| Formula | C6H4N2 |
| Molecular Weight | 128.14 g/mol |
| Melting Point | 141 °C |
| Boiling Point | 304.5 °C (decomposition) |
| Density | 1.238 g/cm3 |
| Apparent Density | ≈ 0.5 g/cm3 |
| Flash Point | 162 °C |
| Heat of Combustion | 4013 kJ/mol |
| Heat of Evaporation | 67 kJ/mol |
| Specific Heat Capacity (30 °C) | 1.30 J g-1 K-1 |
| Vapor Pressure (20 °C) | 0.05 mbar |
| Autoignition Temperature | > 580 °C |
2. Reactions of Phthalonitrile
Phthalonitrile undergoes several important reactions typical of nitrile groups and aromatic compounds, some of which are used in industry.
Phthalonitrile reacts with various metal precursors, such as copper chloride, at a temperature of around 180 °C in a solvent to form intensely colored and durable phthalocyanine pigments.
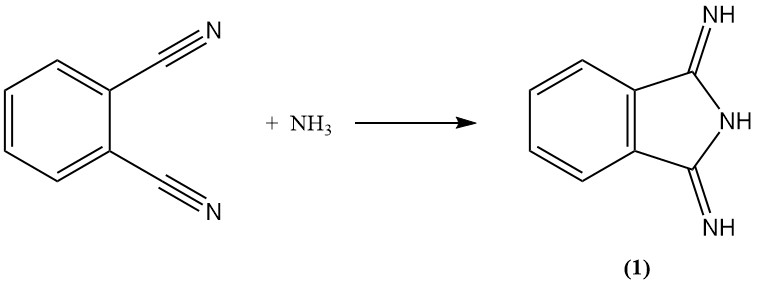
Phthalonitrile reacts with ammonia to form diiminoisoindoline (1). This intermediate can then be further condensed with active methylene compounds to produce commercially important yellow pigments.
Phthalonitrile can participate in curing reactions when combined with specific promoters, like compounds containing sulfhydryl groups. This reaction has potential applications for creating new materials.
Under specific conditions, solid-state reactions can occur between phthalonitrile and other compounds. For example, doping phthalonitrile with diiminoisoindoline can induce thermal reactivity, leading to the formation of phthalocyanine.
At high temperatures above the melting point, phthalonitrile can undergo polymerization, where its individual molecules link to form larger chains, creating a phthalonitrile-based polymer material.
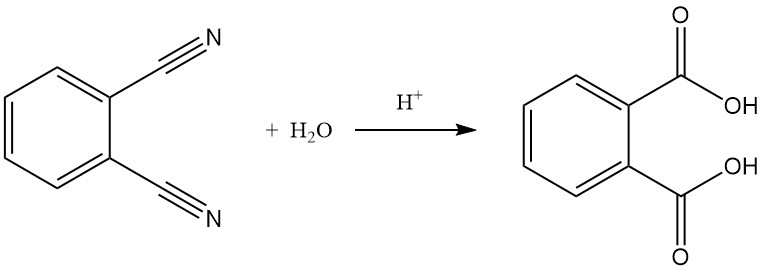
Hydrolysis of phthalonitrile to phthalic acid is possible, especially under harsh conditions like strong acids and elevated temperatures.
Phthalonitrile can undergo limited aromatic substitution reactions such as halogenation, nitration, sulfonation, acylation, and alkylation compared to a simple aromatic ring like benzene because of the deactivating effect of the nitrile groups.
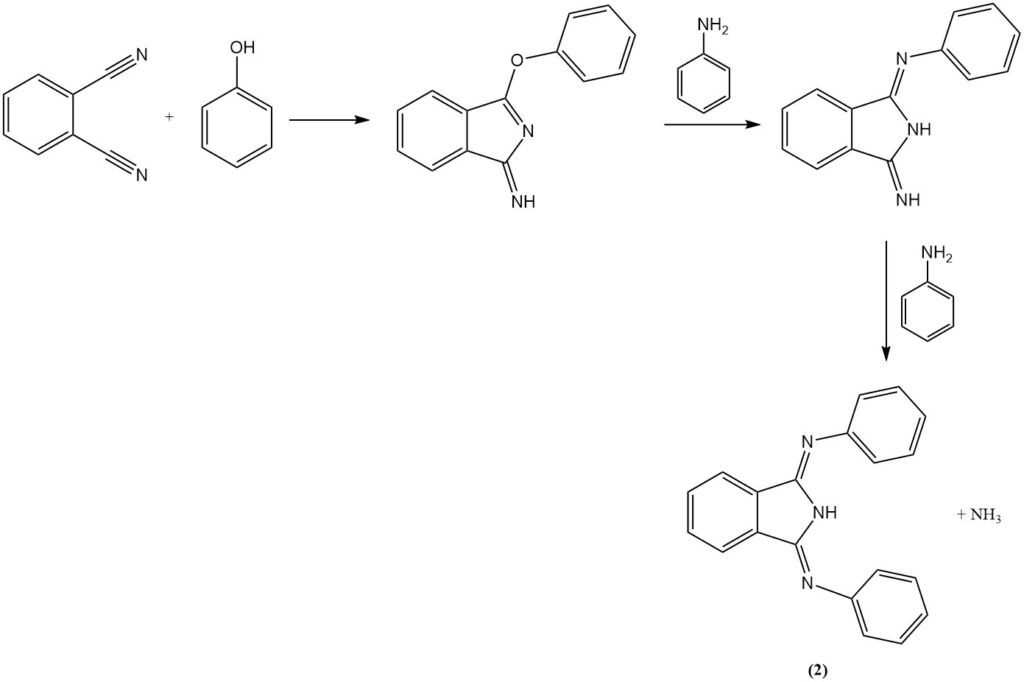
The reaction of phthalonitrile with aniline in the presence of phenol produces a heterocyclic compound (2), and with diamines produces nitrogen-containing macroheterocycles (hexazocyelanes) (3).
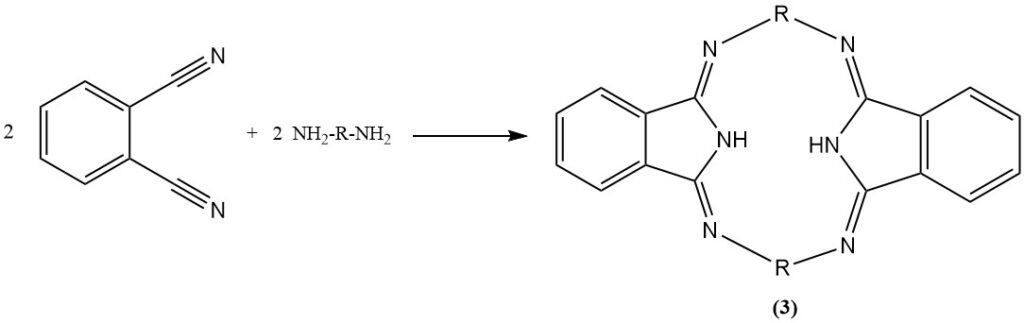
It is also possible to hydrogenate the nitrile groups of phthalonitrile to produce a diamine derivative.
3. Industrial Production of Phthalonitrile
Phthalonitrile is produced commercially from o-xylene, phthalic acid, phthalic anhydride, phthalamide, or phthalimide.
3.1. Production of Phthalonitrile from o-Xylene
Commercially, phthalonitrile is primarily produced from o-xylene by a process called ammoxidation. This single-stage, continuous gas-phase reaction uses a fluidized-bed reactor for efficient conversion.
In the ammoxidation process, metal oxide mixtures containing vanadium, antimony, chromium, and molybdenum supported on alumina or silica are commonly used catalysts. Iron, tungsten, and alkali metal oxides may be added to enhance activity.
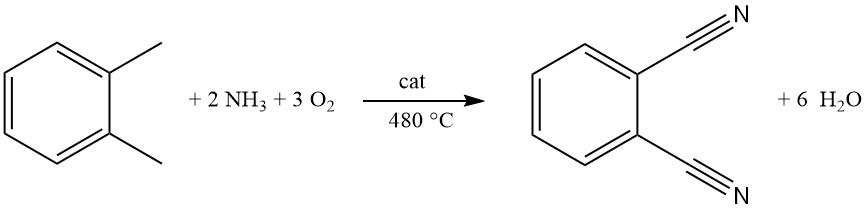
A gaseous mixture of o-xylene, ammonia, and oxygen is fed through a distributor plate into the reactor. The optimal temperature for this pressureless process is around 480 °C.
Lower temperatures increase byproducts like phthalamide and phthalimide (up to 10%), while higher temperatures (>500 °C) lead to ammonia combustion. Integrated cooling systems maintain a constant temperature despite the highly exothermic reaction.
Hot reaction gases are quenched with an aqueous phthalonitrile suspension in a product settler. The cooled mixture is settled and decanted to separate and dry the phthalonitrile product. This high-purity product with acid/phthalimide content < 0.1% and water content < 0.1% can be directly used for phthalocyanine pigment production, achieving yields of 80–85%.
Ammonia is recovered from the post-settler gas stream, which contains NH3, CO2, CO, N2, and trace HCN, using a dedicated ammonia recovery plant. The remaining exhaust gas is combusted, and the recovered ammonia is recycled back into the process.
Unreacted o-xylene and the intermediate o-toluonitrile can be isolated or fed back into the reactor for complete conversion to phthalonitrile.
Pilot plant studies have explored a continuous oxidative ammonolysis process for the synthesis of phthalonitrile from o-xylene. This process uses a vanadium oxide-molybdenum oxide catalyst on alumina or silica support at 350–450 °C.
Here, the metal oxide catalyst acts as the oxidant, with a portion continuously removed, re-oxidized, and recycled. Phthalonitrile quenching and byproduct management are similar to the ammoxidation process.
Byproducts like o-toluonitrile, phthalamide, and phthalimide can be either fed back to the main reactor or converted into phthalonitrile in a separate reactor at a temperature of 400 °C in the presence of ammonia and a boron phosphate-alumina catalyst.
Ammonia is recovered from the residual gas mixture, which contains NH3, CO2, CO, N2, and trace HCN, and recycled back to the reactor. Noxious gases like CO and HCN are combusted in a regenerator, while N2 and CO2 are eliminated from the process stream.
3.2. Production of Phthalonitrile from Phthalic Acid Derivatives
Phthalonitrile can also be synthesized from various phthalic acid derivatives, including phthalic acid, phthalic anhydride, phthalamide, and phthalimide. This process involves a gas-phase reaction with ammonia and water elimination at temperatures ranging from 300 to 500 °C in the presence of a catalyst.
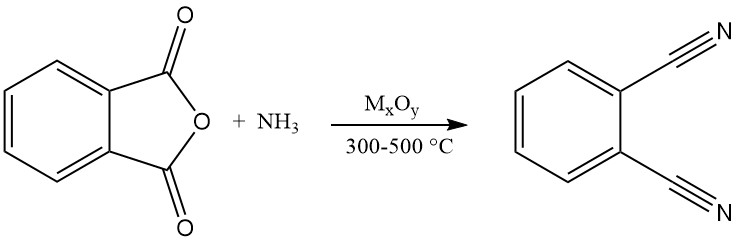
Patent literature suggests the use of metal oxide catalysts supported on silica, alumina, phosphates, silicates, borates, or basic alumina. Examples include oxides of thorium, copper, beryllium, zirconium, or tungsten.
Molten phthalic anhydride is first heated to around 160 °C and vaporized in a dedicated apparatus. Preheated circulating gas containing a high concentration of ammonia (NH3 ≈ 90%) is then introduced into the vaporizer.
This mixture reacts at near-atmospheric pressure at a temperature between 350 and 400 °C in a fixed-bed reactor positioned downstream of the vaporizer. An alumina catalyst is typically used in this reactor.
The hot reaction gas exiting the reactor is quenched with water. Phthalonitrile is then separated from the resulting aqueous suspension by decanting and drying processes. The ammonia concentration in the circulating gas is maintained at around 90% through the continuous addition of ammonia.
Phthalonitrile can be formed by dehydration of phthalamide using acid halides like phosgene or thionyl chloride. However, this method requires additional steps to prevent the hydrolysis of the phthalamide by the generated acid.
Techniques used to overcome this problem include adding diluents (benzene, chlorobenzene) or employing tertiary amines (N,N-diethyl-o-toluidine, pyridine) or acylated secondary amines (N-methylformamide) to neutralize the acid.
This alternative process generally involves complex procedures and additional chemical handling, making it less favorable for large-scale production compared to the established routes.
4. Uses of Phthalonitrile
Phthalonitrile is used as a raw material in the production of phthalocyanine pigments, fluorescent brighteners, and photographic sensitizers.
The production of phthalocyanine pigments, known for their intense colors and exceptional durability, is the most important industrial application of phthalonitrile. Reacting phthalonitrile with various metal precursors gives a wide range of colors. These pigments are used in paints, plastics, textiles, and inks.
5. Toxicology of Phthalonitrile
Acute Toxicity
- Oral LD50 (rat): 30-125 mg/kg.
- No skin or eye irritation was observed in rabbits.
- There is no evidence of sensitization in workers involved in the production and use of phthalonitrile.
Repeated-Dose Toxicity
- Neurotoxic effects (convulsions, increased excitation) were observed in rats and mice following repeated oral exposure.
- Increased activity and body weight decreases were reported in a 13-week rat neurotoxicity study at 10 and 25 mg of phthalonitrile/kg/day. No histopathological link was found for behavioral changes. NOAEL in this study was 3 mg/kg/day.
- Occupational exposure has resulted in delayed (hours to days) epileptiform convulsions lasting minutes in workers.
- Clinical examinations of 81 workers with an average 8.5-year phthalonitrile exposure (including 11 with acute intoxications) showed no abnormalities in clinical chemistry, hematology, neurology, or EEG.
- Dermal exposure may also be relevant.
- Anti-cyanide measures are not considered effective due to a lack of cyanide metabolism.
Genotoxicity
- Phthalonitrile was not mutagenic in the Ames test or the CHO/HGPRT gene mutation assay.
- Negative results in the mouse micronucleus test.
- No increased chromosomal aberrations were observed in workers, including those with acute intoxications.
Carcinogenicity
Studies report leukemia induction in rats and mice after oral, subcutaneous, and percutaneous administration (mice). However, vague study descriptions limit conclusive assessment.
References
- Phthalic Acid and Derivatives, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a20_181.pub2
- https://www.sciencedirect.com/science/article/abs/pii/S0032386118302945
- https://pubchem.ncbi.nlm.nih.gov/compound/Phthalonitrile
- Reaction of O-phthalonitrile with aniline. – https://link.springer.com/article/10.1007/BF00954275


