Benzonitrile is an organic compound with the formula C7H5N. It is a colorless liquid with a sweet bitter almond odor. It is mainly used as a precursor to the resin benzoguanamine.
Table of Contents
1. Physical Properties of Benzonitrile
The following table summarizes the physical properties of benzonitrile.
| Property | Value |
|---|---|
| Formula | C7H5N |
| Molecular mass | 103.1 |
| Color | colorless liquid |
| Odor | almond-like odor |
| Boiling point | 190.7°C |
| Melting point | -12.8°C |
| Relative density (water = 1) | 1.0 g/cm3 (20 °C) |
| Refractive index | 1.53056 (15 °C) |
| Solubility in water, g/100ml at 22°C | 0.1-0.5 (poor) |
| Solubility in organic solvents | Miscible |
| Vapour pressure, Pa at 25°C | 102 |
| Relative vapour density (air = 1) | 3.6 |
| Flash point | 75°C |
| Heat capacity | 1.840 J/g/K |
| Heat of vaporization | 46 kJ/mol |
| Heat of formation | -155 kJ/mol |
| Heat of combustion | 3063 kJ/mol |
| Auto-ignition temperature | 550°C |
| Explosive limits, vol% in air | 1.4-7.2 |
| Octanol/water partition coefficient as log Pow | 1.56 |
2. Chemical Reactions of Benzonitrile
Benzonitrile is a colorless liquid that is stable in air and light.
It is a powerful solvent that dissolves poly(vinyl chloride), poly(vinyl acetate) resins, polystyrene, poly(methacrylate), and nitrocellulose. However, it does not dissolve polyethylene, polyamide, poly(vinyl alcohol), or fluoropolymers.
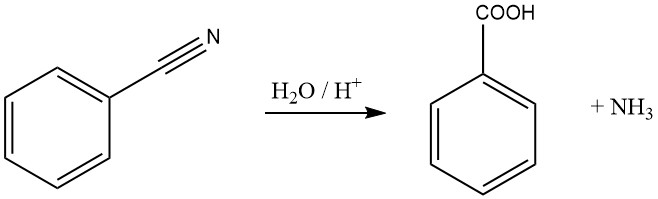
Benzonitrile can be hydrolyzed to benzoic acid and ammonia in the presence of a strong base or acid.
Hydrogenation of benzonitrile produces Benzylamine and dibenzylamine.

The reaction of benzonitrile with a Grignard reagent, such as methylmagnesium bromide (CH3MgBr), in the presence of dry ether gives an addition product, which on acidic hydrolysis yields acetophenone.

The reaction proceeds as follows:
- The Grignard reagent adds to the carbon-nitrogen triple bond in benzonitrile, forming an imine.
- The imine is hydrolyzed by acid, breaking the carbon-nitrogen bond and forming a ketone.
3. Production of Benzonitrile
Benzonitrile is produced by the vapor-phase ammoxidation of toluene, which is the reaction of toluene with ammonia in the presence of oxygen.

The reaction is typically carried out at high temperatures (450 °C) and pressures (10-20 atm) over a catalyst, such as tungsten-manganese complex. The reaction is highly exothermic, with a heat of reaction of -256 kJ/mol.
The traditional catalysts for the ammoxidation of toluene are vanadium and molybdenum, but these catalysts suffer from low selectivity and serious decomposition of ammonia. A tungsten-manganese complex catalyst shows better performance, with a selectivity of up to 87.4%.
The reaction is carried out in a fixed-bed reactor. The concentration of toluene in the feed stream is typically 1.5 vol %. The ratio of ammonia to toluene is 4:1 with a contact time of 2.4 s. The reaction converts 97% of the toluene and 30% of the ammonia. The crude product, which contains ammonia, hydrogen cyanide, toluene, and high-boilers, is purified by distillation.
Benzonitrile can also be produced from benzoic acid and ammonia. This reaction is carried out in the vapor phase at 400-410 °C over alumina or in the liquid phase at 225-245 °C.

Other methods for producing benzonitrile include:
- Liquid-phase ammoxidation of toluene in the presence of a cobalt or manganese bromide catalyst
- Dehydrogenative condensation of benzyl alcohol or benzaldehyde with ammonia
- The high-temperature reaction of toluene with nitrous oxide
- Cyanation of benzene with cyanogen chloride or dicyanogen
4. Uses of Benzonitrile
Benzonitrile is a versatile chemical with a variety of uses. It is a precursor to many other chemicals, including benzoguanamine which is made by the reaction of benzonitrile with dicyanodiamide in the presence of a strong base.
Benzoguanamine reacts with formaldehyde or alcohols to produce a heat-resistant thermosetting resin that is used in a variety of applications, including coatings, adhesives, and composites.
Other uses of benzonitrile include:
- Solvent for a variety of organic compounds
- Reagent in organic synthesis
- Intermediate in the production of pharmaceuticals and pesticides
- Flame retardant
- Flavoring agent
5. Toxicology and Hazards
5.1. Toxicology
Benzonitrile, while exhibiting lower toxicity than aliphatic nitriles, it has the capacity to be absorbed through the skin, which can lead to adverse effects such as tissue convulsions and nerve paralysis.
The following toxicological data provides insight into its potential hazards:
Oral Exposure:
- Rat: LDLo (Lethal Dose, Low) – 720 mg/kg
- Mouse: LD50 (Lethal Dose, 50% mortality) – 971 mg/kg
Inhalation Exposure:
- Rat: LCL0 (Lethal Concentration, Low) – 950 ppm
- Mouse: LC50 (Lethal Concentration, 50% mortality) – 1800 mg/m3
Intraperitoneal (i.p.) Exposure:
- Mouse: LD50 – 400 mg/kg
Subcutaneous (s.c.) Exposure:
- Rabbit: LDL0 (Lethal Dose, Low) – 200 mg/kg
Skin Exposure:
- Rabbit: LD50 – 1250 mg/kg
- Skin Irritation: Moderate
5.2. Hazards
Benzonitrile presents certain acute hazards that require careful consideration:
Fire and Explosion:
The substance is combustible and, in the event of a fire, it releases both irritating and potentially toxic fumes or gases. At temperatures exceeding 75 °C, there is a risk of forming explosive vapor/air mixtures.
Open flames should be strictly avoided. For operations surpassing 75 °C, it is advisable to employ a closed system along with adequate ventilation.
Fire control measures should include the use of suitable extinguishing agents such as powder, AFFF (aqueous film-forming foam), foam, and carbon dioxide. In case of fire, it’s recommended to keep containers cool by applying water spray.
Preventing Mist Generation: It is crucial to take precautions to prevent the generation of mists, as exposure to such mists can lead to adverse health effects.
5.3. Syptoms of Exposure
Exposure to benzonitrile may result in the following symptoms:
Inhalation: Confusion, headache, labored breathing, nausea, unconsciousness, vomiting, and weakness are potential symptoms. Adequate ventilation, local exhaust systems, or personal breathing protection should be employed to mitigate inhalation risks. If exposure occurs, move the affected individual to fresh air and provide rest. In severe cases, artificial respiration might be necessary, and immediate medical attention should be sought.
Skin: Contact with the skin can cause redness. To prevent skin exposure, protective gloves and clothing are recommended. In case of contact, contaminated clothing should be removed, and the affected skin area should be thoroughly rinsed with abundant water or a shower. Medical attention is advised.
Eyes: Redness and pain are potential eye-related symptoms. Proper eye protection, such as safety goggles combined with respiratory protection, should be worn. In case of eye contact, the eyes should be rinsed with copious amounts of water for several minutes. If the individual wears contact lenses, they should be removed before rinsing. Immediate medical attention is necessary.
Ingestion: Ingestion-related symptoms can be similar to those resulting from inhalation exposure. To prevent ingestion, it’s essential not to eat, drink, or smoke during work involving benzonitrile. If ingested, the mouth should be rinsed and the person should rest. Medical attention should be sought promptly.
It is of paramount importance to adhere to appropriate safety measures when working with benzonitrile, including prevention of exposure, proper personal protective equipment (PPE) usage, and prompt medical attention in case of exposure-related symptoms or incidents.
References
- “Benzoic Acid and Derivatives”, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_555


