Crotonic Acid: Properties, Reactions, Production and Uses
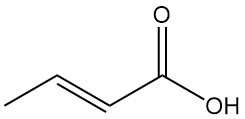
Crotonic acid is the trans-isomer of 2-butenoic acid. It is a short-chain, unsaturated carboxylic acid with the chemical formula CH3CH=CHCOOH. The cis isomer of 2-butenoic acid is known as isocrotonic acid.
Crotonic acid appears as a white to yellowish solid with a pungent, suffocating odor, while isocrotonic acid is an oily, colorless liquid with a characteristic odor resembling brown sugar.The term “crotonic acid” comes from croton oil, an oil derived from the seeds of Croton tiglium. Initially, crotonic acid was mistakenly identified as a product formed during the saponification of this oil.
Crotonic acid can be found in crude wood distillate and is produced as a metabolite during fatty acid degradation.
Table of Contents
1. Physical Properties of Crotonic Acid
When crystallized, crotonic acid forms white needles, or prism crystals, in the monoclinic system. It is slightly soluble in water and soluble in ethanol, acetone, ethyl acetate, and toluene, and forms azeotrope with water containing 96.86% crotonic acid at 99.7 °C.
Isocrotonic acid is miscible with water and polar solvents and forms an eutectic mixture with crotonic acid (30% crotonic acid), which melts at -3 °C.
Table 1 summarizes some of the physical properties of crotonic acid and isocrotonic acid.
| Property | Crotonic acid | Isocrotonic acid |
|---|---|---|
| Molecular weight | 86.09 g/mol | 86.09 g/mol |
| Boiling point | 189 °C | 169°C |
| Melting point | 72°C | 15°C |
| Density (20°C) | 1.018 | 1.0267 |
| Refractive index | 1.4228 (80 °C) | 1.4456 (20 °C) |
| Solubility in water, g/kg | 41.5 (0°C), 94 (25°C) | 656 (40°C), 1260 (42°C) |
| Flash point | 88 °C | - |
| Auto ignition temperature | 396 °C | - |
| Vapor pressure | 24 Pa (20°C), 880 Pa (70°C) | - |
| Specific heat | 3.031 J g-1 K-1 (solid) 2.072 J g-1 K-1 (liquid) |
- |
| Heat of combustion | 2.00 MJ/mol | 2.03 MJ/mol |
| Heat of fusion | 150.9 J/g | - |
| pKa | 4.817 (25°C) | - |
2. Chemical Reactions of Crotonic Acid
Crotonic acid is a weak acid in aqueous solutions, so it can react with organic and inorganic bases.
Crotonic acid undergoes various transformations upon heating, exposure to acids, bases, UV radiation, or other reagents. These reactions include isomerization, oligomerization, and polymerization.
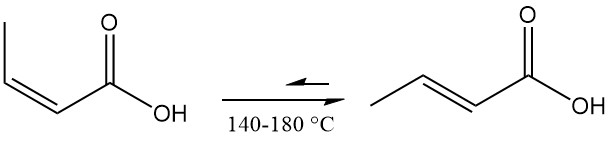
Isocrotonic acid isomerizes to crotonic acid, reaching equilibrium at 140–180 °C. 3-butenoic acid forms as a byproduct, with the final mixture containing an isocrotonic acid-to-crotonic acid ratio of about 0.17:1.
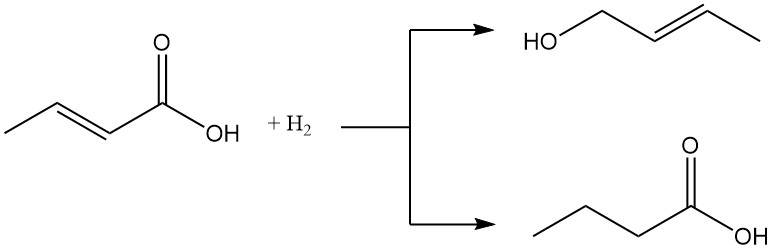
Both crotonic and isocrotonic acids can be reduced to either crotyl alcohol or butyric acid. Conversely, they can be oxidized to the corresponding peracid.
Crotonic acid readily copolymerizes with various monomers via a radical mechanism. Copolymers with vinyl acetate are industrially important.
Addition reactions to the double bond of crotonic acid yield 2-substituted or 2,3-disubstituted butyric acids.
Catalytic hydrogenation converts crotonic acid to butanoic acid.
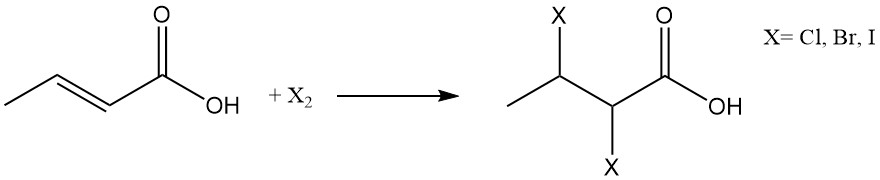
The halogenation or the addition of hydrogen halides leads to the formation of 2,3-dihalobutyric acids or 3-halobutyric acids, respectively.
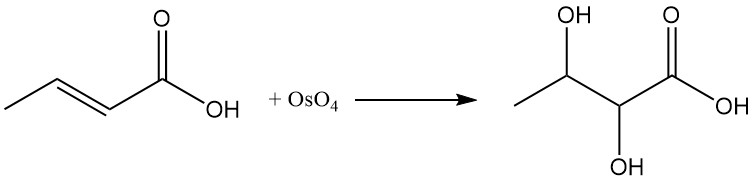
The reaction of crotonic acid with osmium tetroxide (OsO4) or peroxybenzoic acid produces 2,3-dihydroxybutanoic acid.
Under suitable reaction conditions, ammonia addition yields β-aminobutyric acid.
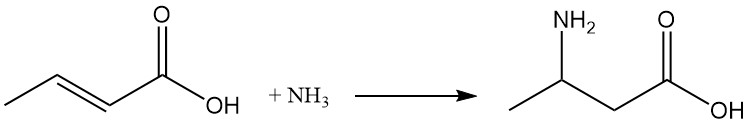
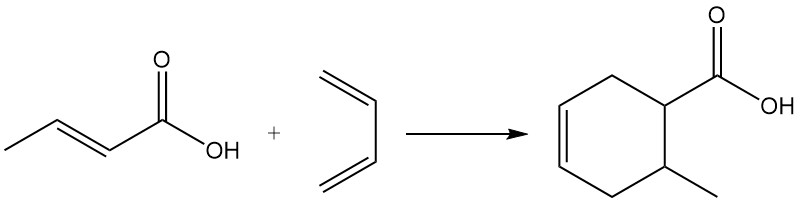
Due to its dienophilic character, crotonic acid participates in Diels-Alder reactions.
Esterification of crotonic acid is possible using conventional methods. However, the presence of the conjugated double bond often leads to slower reaction rates compared to butyric acid.
Crotonyl halides can be synthesized by reacting crotonic acid with the corresponding acyl halide.
Crotonic anhydride is obtained either by the reaction of crotonyl chloride with sodium crotonate or by treating crotonic acid with acetic anhydride.
3. Production of Crotonic Acid
Crotonic acid is industrially produced by the oxidation of crotonaldehyde. This process is used by companies like Weylchem Frankfurt and employs a two-step reaction sequence:
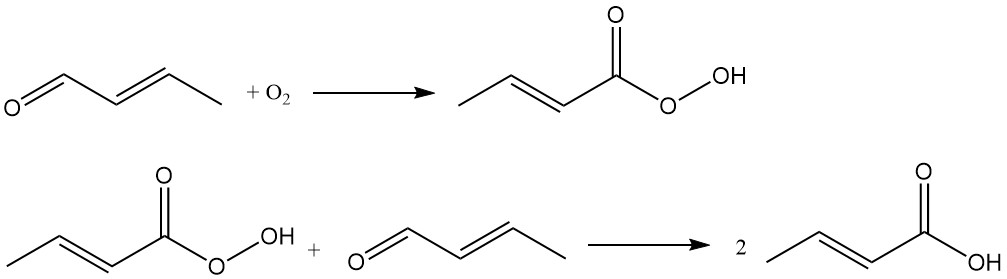
Crotonaldehyde undergoes oxidation to form peroxocrotonic acid as an intermediate, which reacts with another crotonaldehyde molecule to yield crotonic acid.
The reaction typically occurs at moderate temperatures (20–45 °C) and pressures (100–500 kPa). Metal salts, particularly those of manganese, cobalt, copper, or thallium, are added as catalysts to prevent the unwanted accumulation of peroxides. Byproducts include formic acid, acetic acid, water, and carbon dioxide from complete oxidation.
A historical process by Hoechst used a manganese salt catalyst for crotonaldehyde oxidation at 20–30 °C. The resulting reaction mixture contained approximately:
- 20–30% crotonic acid
- 1–3% formic and acetic acid
- 3-5% water
- Trace amounts of 3-butenoic acid, unknown compounds, and 0.5–1% isocrotonic acid
Excess crotonaldehyde is recovered by vacuum distillation and recycled back into the reaction. The distillation residue, rich in crotonic acid (60–70%), undergoes further purification via a second vacuum distillation.
The low-boiling fraction and residue are discarded as waste. The main product fraction contains crotonic acid with some isocrotonic acid (3-5%). Fractional crystallization is then used to remove the isocrotonic acid, yielding high-purity crotonic acid (up to 99.9%).
While industrial production relies on crotonaldehyde oxidation, several alternative methods exist for the laboratory-scale synthesis of crotonic acid. These include:
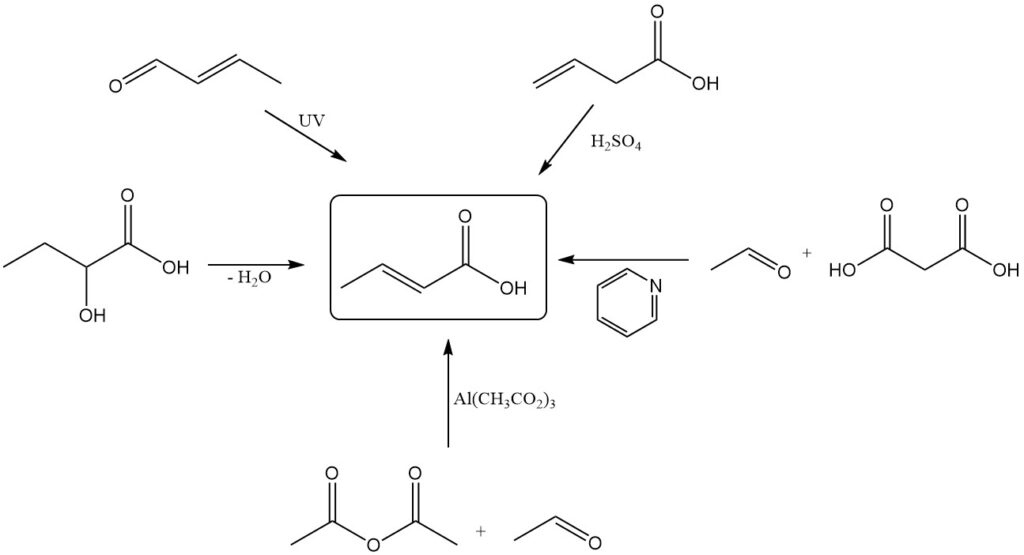
- Dehydration of 2-hydroxybutanoic acid
- Photochemical oxidation or oxidative irradiation (with ultrasound) of crotonaldehyde
- Isomerization of vinylacetic acid with sulfuric acid
- Condensation of acetaldehyde and malonic acid using pyridine as a catalyst
- Oxidation of butene with a heteropolymolybdic acid catalyst system
- Oxycarbonylation of propene with transition metal complex catalysts
- Carbonylation of propylene oxide
- Reaction of acetic anhydride with acetaldehyde using basic aluminum acetate catalyst
- Carbonylation of allyl alcohol with nickel or palladium-based catalysts
Isocrotonic acid can be separated from mixtures containing crotonic acid using techniques like fractional crystallization, rectification (distillation), or gel filtration chromatography.
A stereospecific method for laboratory preparation of isocrotonic acid involves the bromination of 2-butanone (methyl ethyl ketone), followed by Favorskii rearrangement.
4. Uses of Crotonic Acid
The primary application of crotonic acid is the production of copolymers with various comonomers. Crotonic acid-vinyl acetate copolymers are of industrial importance. These copolymers are often marketed under trade names like Mowilith, Vinnapas, and Vinac.
Crotonic acid copolymers are used in paints, coatings, hot-melt paints, adhesives, and hot-melt adhesives. They are also used for coatings on paper and textiles, as flocculants, binders for explosives, ceramics, and agrochemicals, and as drilling additives.
Other applications of crotonic acid include:
- As an antimicrobial agent in ester form for deodorants.
- In UV absorbers and metal-effect interference pigments.
- Fatty alcohol esters of crotonic acid are employed in the leather industry.
- The free acid has applications in motor fuels, metal surface etching, electrochemical metal deposition, and PVC heat stabilization.
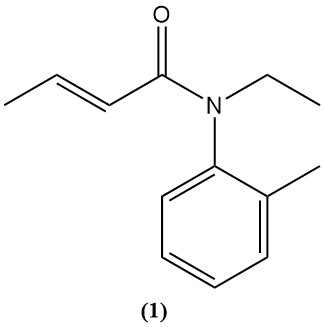
Crotonic acid and its derivatives, such as anhydride and chloride, serve as precursors for various agrochemicals and pharmaceuticals. An example is crotamiton (1), a drug used to treat scabies (scabicidal) and relieve itching (antipruritic).
5. Toxicology of Crotonic Acid
Crotonic acid is a corrosive substance and can cause severe irritation or burns upon contact with the eyes, skin, or respiratory tract. Inhalation can lead to a burning sensation, cough, headache, nausea, sore throat, and shortness of breath (symptoms may be delayed). Ingestion causes pain, burning sensation, sore throat, diarrhea, and vomiting.
Toxicology data from animal studies is listed below:
- LD50 (rat, oral): 1000 mg/kg
- LD50 (rat, i.p.): 100 mg/kg
- LD50 (rabbit, dermal): 600 mg/kg
The body can naturally produce crotonic acid during fat metabolism and rapidly break it down by enzymes in the liver and other tissues. Exposure to non-irritating concentrations is unlikely to cause cumulative effects.
Crotonic acid can affect plant growth and seed germination.
Reference
- Crotonaldehyde and Crotonic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a08_083.pub2
