Chemical Reactions in Industrial Processes

Chemical reactions are a fundamental aspect of many industrial processes. These reactions involve the transformation of one or more substances into new products, and they can be used to produce a wide variety of materials, including fuels, plastics, pharmaceuticals, and many others.
Understanding the principles behind chemical reactions is crucial for engineers and chemists who design and optimize industrial processes.
There are several types of chemical reactions that occur in industrial processes, including combustion, oxidation-reduction reactions, polymerization, hydrogenation, carbonylation, acylation, alkylation, nitration, dehydrogenation, esterification, and sulfonation.
Table of Contents
1. Combustion reactions
Combustion reactions can be classified into two categories: complete combustion and incomplete combustion. In complete combustion, the fuel is burned completely, producing only carbon dioxide and water as the final products.
In contrast, incomplete combustion occurs when the fuel does not burn completely, producing a mixture of carbon monoxide, soot, and other pollutants.

In industrial processes, complete combustion is desirable because it produces the maximum amount of energy and the least amount of pollution. However, achieving complete combustion can be challenging, especially when burning fuels that are difficult to ignite or have low combustion efficiency.
To optimize combustion reactions, several factors must be considered, including the stoichiometry of the reaction, the combustion temperature, and the mixing of the fuel and oxidizing agent.
The stoichiometry of the reaction refers to the ratio of the reactants needed to achieve complete combustion. The combustion temperature is also critical because it affects the rate of the reaction and the quality of the final products.
The mixing of the fuel and oxidizing agent is also essential because it affects the efficiency of the reaction and the quality of the final products.
In addition to industrial processes, combustion reactions are also involved in many natural phenomena, such as wildfires and volcanic eruptions. These reactions can have significant environmental impacts, such as air pollution and global warming.
To reduce the environmental impact of combustion reactions, many efforts are being made to improve the efficiency of combustion and develop cleaner technologies that produce fewer pollutants.
2. Oxidation-reduction reactions
Oxidation-reduction reactions, also known as redox reactions, involve the transfer of electrons between two species. In an oxidation reaction, a species loses electrons, while in a reduction reaction, a species gains electrons.

These reactions are essential in many industrial processes, such as metal production, electroplating, and the production of chemicals and fuels. Important industrial uses of electrochemical reactions are the production of sodium hydroxide by electrolysis of NaCl, the production of other alkali metal hydroxides, and the production of bleach.
Redox reactions can be classified as either spontaneous or non-spontaneous, depending on whether they occur naturally or require energy input to proceed. Spontaneous redox reactions are those that occur without any external energy input and are often used in batteries and other energy storage devices.
Non-spontaneous redox reactions, on the other hand, require an external source of energy to proceed and are used in electrolysis and other chemical processes.
One common example of a redox reaction is the rusting of iron, which was mentioned earlier. In this reaction, iron reacts with oxygen to produce iron oxide, which is the familiar reddish-brown substance we call rust.
Rusting is an example of an oxidation reaction, where iron loses electrons to oxygen. This reaction is important because it is the primary cause of corrosion in metals, which can be costly to repair or replace.
Another example of a redox reaction is the production of chlorine gas, which is used in the production of plastics and other materials. Chlorine gas is produced by the electrolysis of saltwater, which is a non-spontaneous redox reaction that requires an external source of energy to proceed.
In this reaction, chloride ions from saltwater are oxidized to produce chlorine gas and hydrogen gas. Chlorine gas is a potent oxidizing agent and is used in many industrial processes, including the production of pesticides, pharmaceuticals, and plastics.
3. Polymerization Reactions
Polymerization reactions are a type of chemical reaction where monomer molecules are chemically bonded together to form a polymer chain. The resulting polymer can have a wide range of properties and applications, from plastics and synthetic fibers to adhesives and coatings.

There are two main types of polymerization reactions: addition polymerization and condensation polymerization. In addition polymerization, monomers with a double bond react with each other to form a polymer chain.
The reaction is typically initiated by a catalyst or heat, and the resulting polymer is a long chain with repeating units of the original monomer. Condensation polymerization, on the other hand, involves the reaction of two different monomers with a functional group that reacts to form a covalent bond. In this type of reaction, a small molecule, such as water, is typically produced as a byproduct.
Polymerization reactions are used in a wide range of industrial applications, from the production of plastics and synthetic fibers to the development of new materials for medical implants and drug delivery.
One example of a polymerization reaction is the production of polyethylene, which is the most widely used plastic in the world. Polyethylene is produced by the addition polymerization of ethylene monomers, which results in a long-chain polymer that can be used in a wide range of applications, from packaging to building materials.
Another example of a polymerization reaction is the production of nylon, which is a synthetic fiber used in textiles and other applications. Nylon is produced by the condensation polymerization of two monomers, hexamethylenediamine and adipic acid, which react to form a polymer chain. Nylon is known for its strength and durability and is used in a wide range of applications, from clothing to automotive parts.
4. Oxidation Reactions
An oxidation reaction is one where a molecule loses electrons. This often goes hand-in-hand with the gain of oxygen atoms, but not always. Here’s a breakdown of the key aspects:
- Electron Transfer: The core principle of oxidation is the loss of electrons from a molecule. Think of it as removing negative charges, making the molecule more “positive” in charge.
- Oxygen Connection: While often associated with gaining oxygen atoms, an oxidation reaction doesn’t strictly require it. However, oxygen is a common oxidizer due to its strong electronegativity, meaning it has a high affinity for attracting electrons.
Different oxidizers used in the chemical industry, like molecular oxygen (O2), hydrogen peroxide (H2O2), and chlorine (Cl2), accept electrons from the target molecule. The choice of oxidizer depends on factors like cost, selectivity, and reaction conditions.
Industrial catalysts speed up the reaction without being consumed themselves. Transition metals like copper, manganese, and vanadium are frequently used. They can enhance selectivity, reduce energy consumption, and improve process efficiency.
The oxidation reactions are used in different industries:
1. Production of bulk chemicals:
- Acrylic acid is important for acrylic fibers, paints, and adhesives. It’s produced by propylene oxidation with air over metal oxide catalysts.
- Terephthalic acid is the building block of PET plastics; it’s manufactured by liquid-phase oxidation of p-xylene using air or oxygen.
- Nitric acid is used in fertilizers, explosives, and nylon production. It’s synthesized via the Ostwald process, involving ammonia oxidation with air over platinum-rhodium catalysts.
2. Fine chemicals and pharmaceuticals:
- Selective oxidations: precisely altering specific functional groups in complex molecules is crucial for synthesizing pharmaceuticals. Enzymes or transition metal catalysts enable targeted oxidations with minimal side reactions.
- Hydroxylations: Introducing hydroxyl groups is vital for many pharmaceuticals. Methods like Sharpless asymmetric dihydroxylation utilize chiral catalysts for high stereocontrol.
3. Environmental remediation:
- Wastewater treatment: Oxidation with ozone or peroxides effectively disinfects water and degrades organic pollutants.
- Flue gas desulfurization: Removing sulfur oxides from power plant emissions involves wet scrubbing with oxidants like calcium hydroxide.
5. Hydrogenation Reaction
A hydrogenation reaction is a specific type of reduction reaction where a molecule gains hydrogen atoms. This often occurs in the presence of a catalyst and results in the saturation of unsaturated bonds, typically double or triple bonds, in the reacting molecule.
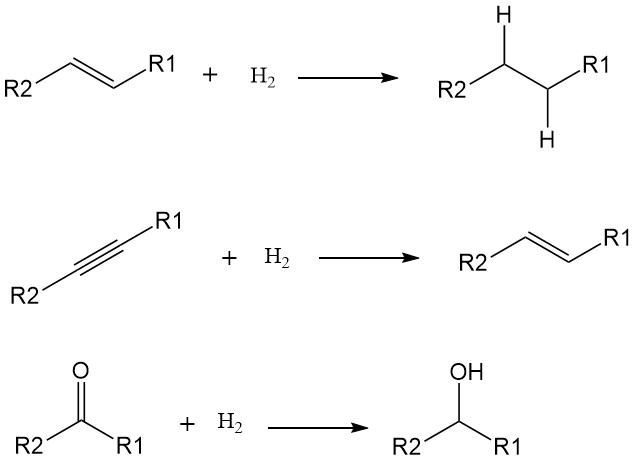
The addition of hydrogen atoms (H) to a molecule increases its saturation and reduces its reactivity. While some hydrogenations can occur at high temperatures without catalysts, their use significantly lowers the activation energy and speeds up the reaction under milder conditions. Transition metals like nickel, palladium, and platinum are common catalysts for hydrogenation.
Hydrogenations typically target double and triple bonds, converting them into single bonds. Examples include:
- Alkenes to alkanes: Transforming unsaturated hydrocarbons like ethylene (C2H4) into saturated hydrocarbons like ethane (C2H6).
- Alkynes to alkenes: converting triple-bonded hydrocarbons like acetylene (C2H2) into double-bonded alkenes like ethylene.
- Carbonyl groups to alcohols: Reducing ketones and aldehydes with carbonyl groups (C=O) to alcohols with hydroxyl groups (OH).
Hydrogenation reactions are used in various industries, including:
- Vegetable oil processing: hardening liquid oils by converting unsaturated fats to saturated fats for margarine production.
- Fuel refining: desulfurizing and improving the quality of gasoline and diesel fuels.
- Pharmaceutical production: synthesizing various pharmaceuticals and fine chemicals.
Hydrogenation is also used in:
- Food and beverage industry: processing fats and oils for food products.
- Polymerization: creating polymers with specific properties.
- Hydrogen fuel cells: converting hydrogen and oxygen into electricity.
6. Carbonylation Reaction
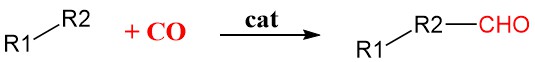
Carbonylation is a general method in organic synthesis that adds carbon monoxide (CO) into organic molecules using transition metal catalysts such as rhodium, iridium, palladium, or nickel. These catalysts bind and activate the CO molecule, making it more reactive and allowing it to be incorporated into the organic substrate.
It’s a highly selective reaction, meaning it can precisely add the CO molecule to the desired position in the molecule with minimal side reactions.
This makes it valuable for producing a wide range of important chemicals, including:
- Acetic acid: Over 70% of the world’s synthetic acetic acid is produced using rhodium- or iridium-catalyzed methanol carbonylation.
- Amino acid derivatives: Amidocarbonylation is a growing area of interest for creating valuable amino acid derivatives used in pharmaceuticals and other industries.
- Pharmaceutical intermediates: carbonylation of aromatic halides is emerging as a standard procedure for creating important building blocks for drugs and other bioactive molecules.
7. Acylation Reaction
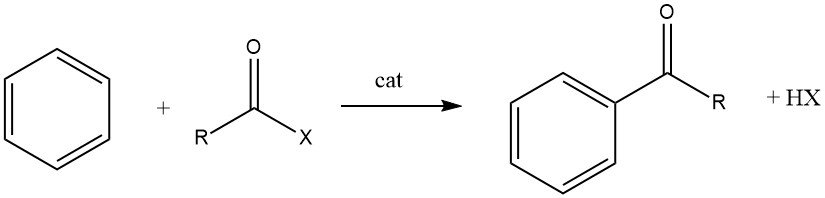
The Friedel-Crafts acylation involves the production of an aromatic ketone by the reaction between an aromatic compound and an acylating agent, which could be an acyl halide, an acid anhydride, an acid, or an ester. This reaction takes place in the presence of an acidic catalyst.
The acylation of aromatic substrates holds significant industrial importance as it is utilized for the synthesis of aromatic intermediates extensively employed in the manufacturing of pharmaceuticals, insecticides, plasticizers, dyes, perfumes, and various other commercial products. These specialty compounds are typically produced on a smaller scale compared to alkylated products.
| Acylating agent | Aromatic compound | Product | End use |
|---|---|---|---|
| Acetic anhydride | benzene | acetophenone | perfumes, pharmaceuticals, solvent, plasticizer |
| Acetic anhydride | toluene | 4-methylacetophenone | perfumes |
| Acetic anhydride | anisole | 4-methoxyacetophenone | perfumes |
| Acetic anhydride | isobutylbenzene | 4-isobutylactophenone | pharmaceuticals |
| Dichloroacetyl chloride | 1,2-dichlorobenzene | α,α,2,4-tetrachloroacetophenone | insecticides |
| Chlorobutyroyl chloride | fluorobenzene | chloropropyl 4-fluorophenyl ketone | pharmaceuticals |
| Tetrachloromethane | benzene | benzophenone | pharmaceuticals, insecticides, perfumes |
| Benzoyl chloride | benzene | benzophenone | |
| Phosgene | N,N-dimethylaniline | 4,4'-bis-dimethylaminobenzophenone | dyes |
| Phthalic anhydride | benzene | 2-benzoylbenzoic acid | anthraquinone |
8. Alkylation Reaction
The Friedel-Crafts alkylation of aromatic compounds involves an acid-catalyzed electrophilic substitution, wherein an alkyl group replaces an aromatic hydrogen. A diverse range of alkylating agents, such as olefins, alkyl halides, and alcohols, is commonly employed for this purpose.
This reaction can be applied to various aromatic substrates, including heteroaromatic compounds and even compounds like ferrocene. These reactions are typically rapid and exothermic, and they are often conducted under mild conditions in the liquid phase.
However, in some cases, vapor-phase processes with more stringent conditions are utilized for certain substrates. When olefins, alkyl halides, and alcohols are used as alkylating agents, the overall reactions proceed as follows:
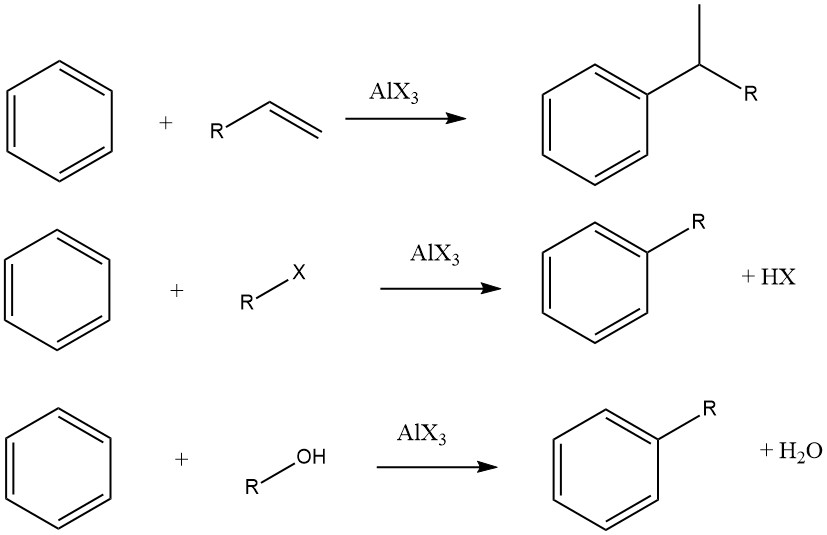
The degree of isomerization of the alkyl moiety and adherence to the Markovnikov rule during the addition process vary depending on the acidity of the catalyst.
Examples of uses of alkylation reactions are the production of ethylbenzene, cumene, and alkylbenzenes from benzene, the alkylation of toluene with propene to produce cymene; the alkylation of phenols to produce alkylphenols; and the alkylation of various heterocyclic aromatic compounds, including furans, thiophenes, and N-heterocycles.
9. Nitration Reaction
The reaction of nitration is the irreversible introduction of one or more nitro (NO2) groups into an aromatic nucleus, replacing a hydrogen atom.
Nitration is an electrophilic substitution reaction, represented by the following equation:
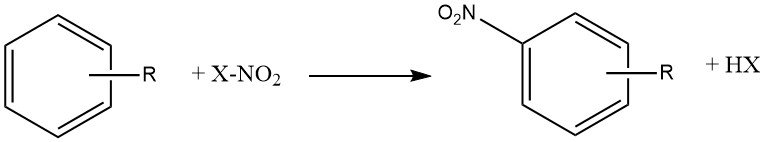
The introduction of the nitro group deactivates the ring towards further electrophilic substitution, making dinitration a rare occurrence under the conditions used for mononitration.
More vigorous conditions are usually required for dinitration, necessitating excess, stronger acid, and higher temperature. These conditions are often applied to isolated mononitro compounds, rather than carrying out stepwise reactions in situ.
The nitration reaction is highly exothermic, as evidenced by the mononitration of benzene (ΔH = -117 kJ/mol) and naphthalene (ΔH = -209 kJ/mol). Consequently, it is one of the most potentially hazardous industrially operated unit processes.
The primary products of nitration are mono-substituted nitroaromatic compounds, which are formed when one nitro group is introduced into the aromatic nucleus. Important industrial products from nitration can include:
- Nitrobenzene (C6H5NO2)
- Nitrotoluenes (e.g., ortho-nitrotoluene, meta-nitrotoluene, and para-nitrotoluene)
- Nitronaphthalenes (e.g., alpha-nitronaphthalene and beta-nitronaphthalene)
- Nitrophenols (e.g., ortho-nitrophenol, meta-nitrophenol, and para-nitrophenol)
- Nitroanilines (e.g., ortho-nitroaniline, meta-nitroaniline, and para-nitroaniline)
10. Dehydrogenation Reaction
Dehydrogenation, the opposite of hydrogenation, refers to a chemical reaction where hydrogen (H) is removed from a molecule, usually organic. This seemingly simple process plays a crucial role in various aspects of chemistry, from fundamental laboratory reactions to large-scale industrial processes.
This reaction involves breaking a C-H bond, releasing H2 gas, and is often endothermic, requiring heat or specific catalysts to overcome the activation energy barrier.
Dehydrogenation is an important reaction in the chemical industry used for:
1. Production of valuable chemicals: Dehydrogenation is essential for making numerous products, like:
- Styrene: monomer for polystyrene plastics, produced from ethylbenzene dehydrogenation.
- Alkenes: unsaturated hydrocarbons used for further reactions in polymer, fuel, and lubricant production.
- Fine chemicals: Pharmaceuticals, agrochemicals, and other specialized molecules often involve dehydrogenation steps.
2. In the energy sector, catalytic dehydrogenation of light hydrocarbons offers potential for cleaner fuel production compared to traditional methods.
3. Dehydration: Removing water (H2O) from alcohols is a special case of dehydrogenation, forming alkenes by eliminating a molecule of water.
11. Esterification Reaction
Esterification is the reversible reaction between carboxylic acids and alcohols to produce an ester and water in the presence of a catalyst.
The esterification reaction proceeds through a three-step mechanism:
- Protonation: A Brønsted acid catalyst protonates the carbonyl oxygen of the carboxylic acid, enhancing its electrophilic character.
- Nucleophilic Attack: The oxygen atom of the alcohol, acting as a nucleophile, attacks the electrophilic carbonyl carbon, forming a tetrahedral intermediate.
- Deprotonation and Water Loss: The intermediate undergoes deprotonation, often by the catalyst, followed by water elimination, leading to the formation of the ester and water as the byproduct.
Esterification is an equilibrium reaction, and Le Chatelier’s principle dictates strategies to enhance ester yield. Employing excess alcohol, removing water (e.g., via distillation), and optimizing temperature can all shift the equilibrium towards ester formation.
The esterification reaction has widespread applications:
- Fragrances and Cosmetics: Esters contribute significantly to the pleasant aromas of fruits, flowers, and perfumes.
- Pharmaceuticals: Numerous drugs, including aspirin and penicillin, incorporate ester functionalities.
- Polymers: Polyesters, a class of polymers, are formed via esterification reactions and find use in various plastics and synthetic fibers.
- Biofuels: Esterification is employed in the production of biofuels like biodiesel.
- Food Additives: Esters contribute to the texture and flavor of certain food products.
12. Sulfonation Reaction
Sulfonation is a versatile organic reaction that introduces the sulfonic acid group (-SO2OH) onto aromatic compounds. This transformation holds significant importance in various fields, including the synthesis of detergents, dyes, and pharmaceuticals.
Sulfonation typically proceeds through an electrophilic aromatic substitution (EAS) mechanism. The key steps involve:
- Formation of the electrophile: Sulfur trioxide (SO3) or fuming sulfuric acid (H2SO4 + SO3) acts as the sulfonating agent, generating the electrophilic SO3 molecule.
- Aromatic electrophilic substitution: The SO3 electrophile attacks the electron-rich π-cloud of the aromatic ring, forming a σ-complex intermediate.
- Protonation and desulfonation: The intermediate loses a proton, leading to the formation of the arylsulfonic acid product.
However, sulfonation is often reversible. Under acidic conditions and elevated temperatures, the sulfonic acid group can undergo desulfonation, releasing the parent aromatic compound and sulfur dioxide.
Sulfonation finds numerous applications in the synthesis of various industrially and biologically relevant molecules:
- Detergents: Sodium dodecylbenzenesulfonate (SDS), a widely used anionic surfactant, is produced by sulfonating dodecylbenzene.
- Dyes: Sulfonated azo dyes exhibit vibrant colors and are employed in diverse textile applications.
- Pharmaceuticals: Sulfonamides, a class of antibiotics, rely on the presence of a sulfonic acid group for their antibacterial activity.
Beyond these examples, sulfonation plays an important role in the synthesis of numerous other compounds, including ion-exchange resins, food additives, and agrochemicals.
References
- Callister, W. D., & Rethwisch, D. G. (2018). Materials Science and Engineering: An Introduction. Wiley.
- Chang, R. (2010). Chemistry (10th ed.). McGraw-Hill.
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford University Press.
- Green, J. (2018). An Introduction to the Chemical Process Industry. CRC Press.
- Kiehl, J. T. (2013). Industrial Chemical Processes: An Introduction. John Wiley & Sons.
- Meyers, R. A. (Ed.). (2001). Encyclopedia of Analytical Chemistry. Wiley.
- Seader, J. D., & Henley, E. J. (2011). Separation Process Principles. Wiley.
- Smith, J. M., Van Ness, H. C., & Abbott, M. M. (2005). Introduction to Chemical Engineering Thermodynamics (7th ed.). McGraw-Hill.
- Sperling, L. H. (Ed.). (2018). Introduction to Physical Polymer Science (4th ed.). Wiley.
- Turton, R., Bailie, R. C., Whiting, W. B., & Shaeiwitz, J. A. (2018). Analysis, Synthesis, and Design of Chemical Processes (5th ed.). Pearson.
