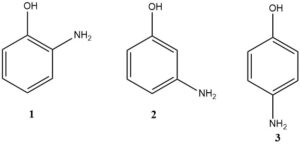
Aminophenol: Properties, Production, Reactions and Uses
Aminophenols have been gaining significant commercial importance, both as standalone substances and as crucial components in the chemical and dye sectors.

Aminophenols have been gaining significant commercial importance, both as standalone substances and as crucial components in the chemical and dye sectors.

Aromatic amines are produced by three types of reactions:
Reductions: using metallic elements like Iron (Fe), Zinc (Zn), Tin (Sn), Aluminum (Al), or their corresponding salts; sulfur-containing compounds; electrochemical procedures; and catalytic hydrogenation.
Nucleophilic substitutions: involving the exchange of substituents like halogen, hydroxyl, alkoxy, and sulfonic groups.
Rearrangements and degradations: including transformations such as the benzidine and Beckmann rearrangements, along with the Schmidt and Hofmann degradations.
It should be noted that the first two reaction types are more important. Chemical rearrangements and degradations rarely result in pure reaction products with high yields.

The primary method of producing aldehydes is oxo synthesis, achieved by mild oxidation (dehydrogenation) of primary alcohols and specialized olefin oxidation processes. In the essential oils of various plants, trace amounts of aldehydes occur naturally. Acetaldehyde, a byproduct of alcohol fermentation, forms by the decarboxylation of the intermediary pyruvic acid.

1,4-butanediol (often abbreviated as 1,4-BDO) is a chemical compound with the molecular formula C4H10O2. It is a colorless and odorless liquid that belongs to the family of diols, which are compounds that contain two hydroxyl (OH) groups.

Production of aliphatic alcohols occurs by various industrial processes, some of which are listed below:
Synthesis from carbon monoxide and hydrogen (C1)
Oxo synthesis, often accompanied by hydrogenation of initially formed aldehydes (C3 - C20)
Hydrogenation of aldehydes, carboxylic acids, or esters
Aldol condensation of lower aldehydes followed by hydrogenation of the alkenals (C3 → C6, C4 → C8, C8 → C16)
Oxidation of trialkylaluminum compounds (Ziegler process)
Oxidation of saturated hydrocarbons
Hydration of olefins (C2–C4)
Homologation of alcohols
Hydrocarbonylation by the Reppe process
Hydrocarboxymethylation
Fermentation processes (C2–C5)
Guerbet process
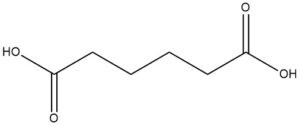
Adipic acid, also known as hexanedioic acid or 1,4-butanedicarboxylic acid, is an organic compound with the formula C6H10O4. It is a widely used aliphatic dicarboxylic acid that appears as a white crystalline solid.
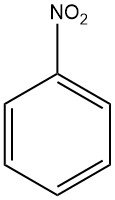
Nitrobenzene is an organic chemical compound with the molecular formula C6H5NO2. It is a pale yellow liquid with a distinctive odor similar to bitter almonds.

The nitration reaction is a chemical process in which one or more nitro (NO2) groups are introduced into an aromatic nucleus by replacing a hydrogen atom. It is an electrophilic substitution reaction commonly used to modify aromatic compounds by attaching nitro groups, which can significantly alter their properties and reactivity.

The Friedel-Crafts acylation involves the production of an aromatic ketone by the reaction between an aromatic compound and an acylating agent, which could be an acyl halide, an acid anhydride, an acid, or an ester. This reaction takes place in the presence of an acidic catalyst.

The Friedel-Crafts alkylation of aromatic compounds involves an acid-catalyzed electrophilic substitution, wherein an alkyl group replaces an aromatic hydrogen. A diverse range of alkylating agents, such as olefins, alkyl halides, and alcohols, is commonly employed for this purpose.
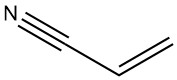
Acrylonitrile is a chemical compound with the chemical formula C3H3N. It is a clear, colorless liquid at room temperature and possesses a nitrile functional group attached to a carbon-carbon double bond. Acrylonitrile is an essential intermediate in the chemical industry and serves as a building block for various products.
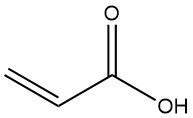
Acrylic acid, also known as 2-propenoic acid, is the simplest unsaturated carboxylic acid with the formula CH2=CHCOOH. It is a colorless liquid that is flammable, volatile and slightly toxic.