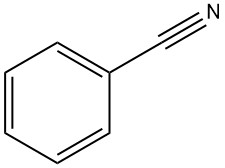
Benzonitrile: Properties, Production and Uses
Benzonitrile is an organic compound with the formula C7H5N, abbreviated PhCN. It is a colorless liquid with a sweet bitter almond odor. It is mainly used as a precursor to the resin benzoguanamine.

Benzonitrile is an organic compound with the formula C7H5N, abbreviated PhCN. It is a colorless liquid with a sweet bitter almond odor. It is mainly used as a precursor to the resin benzoguanamine.

Aluminates are metal salts of alumina (aluminum oxide, Al2O3). The most important aluminates in industry are sodium aluminate and barium aluminate.

Hans Christian Oersted first prepared anhydrous aluminum chloride in 1825 by passing chlorine gas through a heated mixture of alumina and carbon. This compound is a significant catalyst in organic chemistry, particularly for Friedel-Crafts alkylation and acylation, which are used to produce alkylated aromatics, dyestuffs, pharmaceuticals, and perfumery chemicals.

Alum, recognized as a double salt of potassium and aluminum sulfates, was known among the ancient Greeks and Romans as both an astringent and a mordant for wool dyeing. It found diverse application, extending to skin processing, preservation of both animal and human remains, and for fireproofing wood.

Calone 1951 or watermelon ketone is a synthetic organic compound with the formula C11H12O2. It is a white powder with a fresh, marine odor. Watermelon ketone is used in the fragrance industry to create fresh aquatic marine notes.

Aluminum sulfate is a white, odorless, crystalline solid. It is a chemical compound with the formula Al2(SO4)3. It is soluble in water and forms a clear solution. Aluminum sulfate is a common industrial chemical and is used for a variety of purposes.
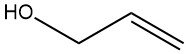
Allyl alcohol is an organic compound with the chemical formula C3H6O. It belongs to the class of alcohols and contains both a hydroxyl group (-OH) and an allyl group (-CH2CH=CH2). It is an important chemical intermediate used in various industries.

Allyl chloride, also known as 3-chloropropene, is an organic compound with the chemical formula C3H5Cl. It is a colorless to pale yellow liquid that holds significance in the field of organic chemistry due to its reactivity and industrial applications.

Ammonium chloride (NH4Cl) is a chemical compound composed of ammonia (NH3) and hydrochloric acid (HCl). It exists as a white crystalline solid with a characteristic salty taste and is commonly known as sal ammoniac.

Ammonium nitrate (NH4NO3) is a chemical compound composed of ammonium ions (NH4+) and nitrate ions (NO3-). It is a colorless, crystalline substance widely used in various applications, including agriculture, industry, and explosives.
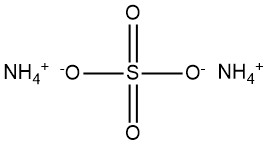
Ammonium sulfate is a chemical compound with the formula (NH4)2SO4. It is commonly encountered as a white, crystalline powder or granular substance. It has emerged as a significant compound during the 19th century produced from ammonia found in coke-oven gas.
4-(N-Methylamino)phenol, 4-(N,N-Dimethylamino)phenol, 4-Hydroxyacetanilide, 4-Methoxyaniline, 4-Ethoxyaniline, 4-Ethoxyacetanilide, 5-Aminosalicylic Acid, N-(4-Hydroxyphenyl)glycine, 4-Amino-2,6-dichlorophenol