Industrial Production of ε-Caprolactam
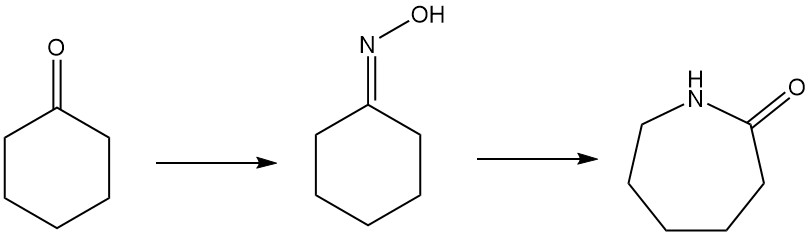
Caprolactam, the key ingredient in Nylon-6, can be produced by several routes. The most common method is the convertion of cyclohexanone to cyclohexanone oxime by ammoximation or hydrogen peroxide processes. This oxime then undergoes a Beckmann rearrangement to form caprolactam.
Other processes include production by photooximation and toluene-based route or is recovered from Nylon-6 waste. Innovative processes are emerging, including the ALTAM route utilizing butadiene or adiponitrile-based methods derived from renewable resources like furfural or biomass.
Modern caprolactam plants are complex, multistage operations, with current single-line capacities reaching an impressive 200,000 tons per year for the widely licensed Fibrant HPO technology.
While cyclohexanone dominates as the starting material (>98%), cyclohexane and toluene can also be used. Notably, phenol hydrogenation and various oxidation pathways for cyclohexane are employed to obtain the key intermediate. The combined efficiency of converting cyclohexanone to caprolactam is approximately 98%.
Table of Contents
1. Production of Caprolactam via Cyclohexanone Process
Modern caprolactam production relies on benzene extracted from BTX streams as the primary feedstock. All commercial processes except one use cyclohexanone oxime as an intermediate. This oxime is formed via the reaction of cyclohexanone with hydroxylamine, generated through various methods like:
- Raschig process
- Nitric oxide hydrogenation in sulfuric acid process
- Fibrant HPO process
- Ammoximation process
1.1. Cyclohexanone Oxime by Raschig Process
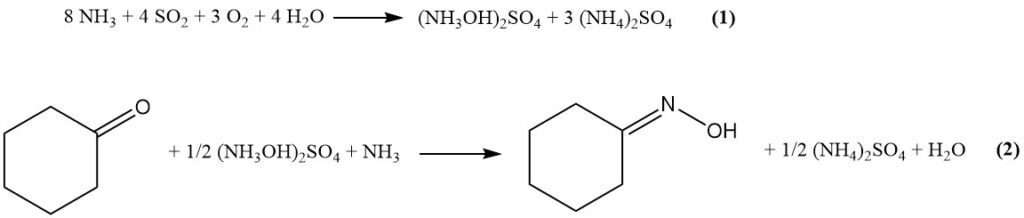
In this process, the cyclohexanone oxime is produced by the reaction of cyclohexanone and hydroxylamine sulfate, and ammonia is used to neutralize the oxime salt. Ammonium sulfate is produced as a byproduct of this reaction.
Several improvements, known as Raschig processes, were implemented to enhance the yield of cyclohexanone oxime and minimize sulfate salt production. Modified Raschig processes, the only ones still in operation, exclusively generate ammonium sulfate as a byproduct.
In the modified Raschig process, first the raw materials (ammonia, CO2, SO2, water, and air) react to form hydroxylamine sulfate via Equation (1). Then, hydroxylamine sulfate reacts with cyclohexanone to produce cyclohexanone oxime (Equation (2)).
The main process steps involved are listed below:
Ammonia absorbs CO2 in water to form an ammonium carbonate/bicarbonate solution.
Ammonia combusts with air (Pt/Rh catalysts) at ~850 °C to generate NOx and water. The hot gases are cooled, recovering exothermic oxidation heat and condensing byproduct dilute nitric acid.
Nitrous gas mixture and ammonium carbonate/bicarbonate solution react at <10 °C and pH 15 to form ammonium nitrite.
Sulfur burning produces SO2 (10–20 vol%), with heat recovery for steam generation.
- SO2 reacts with an aqueous mixture of ammonium nitrite and ammonium hydroxide to form hydroxyimidodisulfuric acid. Temperature and the NH4OH/NH4NO2 ratio are important parameters to minimize byproducts.
Hydroxyimidodisulfuric acid undergoes hydrolysis and neutralization to form hydroxylamine sulfate via ammonium N-hydroxysulfamate.
Oximation of cyclohexanone with hydroxylamine sulfate at pH 4.5 and 50–100 °C yields cyclohexanone oxime.
Aqueous ammonia neutralizes liberated sulfuric acid, and coproduced ammonium sulfate is recovered through evaporative crystallization.
Other byproducts of the Raschig process are nitrous gases in nitrite formation off-gases, nitrogen gas from ammonium nitrite decomposition and N2O gas from various decomposition reactions.
1.2. Nitric Oxide Hydrogenation in Sulfuric Acid Process
To minimize the coproduction of ammonium sulfate, BASF, Cyclopol, and Inventa developed processes involving catalytic nitric oxide reduction with hydrogen to hydroxylamine sulfate in diluted sulfuric acid. Nitric oxide itself is generated via catalytic ammonia oxidation with pure oxygen and steam.
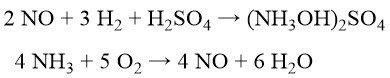
Subsequent oxime production is similar to the Raschig process (Equation (2)). This route, with the neutralization of excess H2SO4, still generates ~0.7 kg of ammonium sulfate per kg of cyclohexanone oxime. Explosive risks are mitigated by the controlled gas composition during hydroxylamine sulfate synthesis. Extensive raw material purification and complex catalyst workups are required.
An ~14% ammonia, ~1.34 O2/NH3 ratio, and steam mixture pass over a Pt/Rh catalyst at ~850 °C to form mainly NO and other nitrogen oxides. The hot gas cools in a waste-heat boiler (generating steam), then further cools to condense water and dilute nitric acid.
The remaining NO2 is washed out in consecutive towers with diluted nitric acid and caustic soda solutions, achieving ~95% purity.
The hydroxylamine synthesis comprises multiple lines of 5-7, 20 m3 well-stirred reactors operated continuously. Each reactor receives a 20 wt% purified sulfuric acid solution with ~35 g/L of finely dispersed Pt-graphite catalyst (~0.5 wt%). All reactors receive purified NO and excess hydrogen. The reaction temperature is ~45 °C.
While atmospheric pressure is used, elevated pressure can be advantageous. A ~25 wt% hydroxylamine sulfate solution containing the catalyst exits the last reactor and undergoes filtration. The concentrated catalyst slurry recycles into the first reactor.
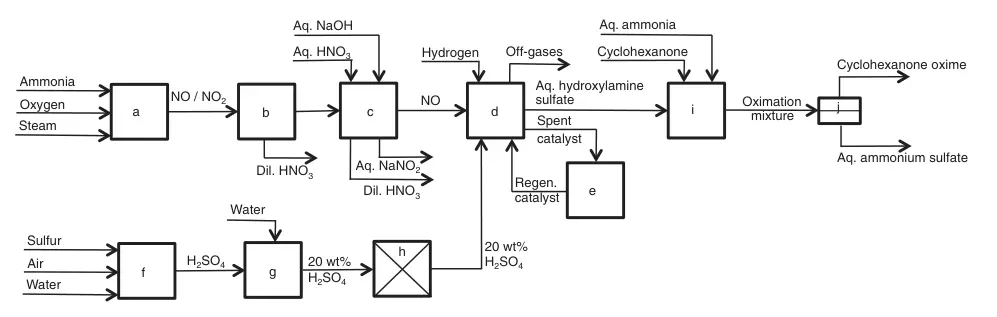
a) Ammonia combustion; b) Condensation; c) NO washing; d) NO reduction; e) Catalyst regeneration; f) Sulfur combustion; g) Dilution; h) Purification; i) Cyclohexanone oximation; j) Cyclohexanone oxime/aqueous ammonium sulfate separation
Extremely corrosive reaction mixtures and catalyst sensitivity necessitate careful material selection. Inventa proposed rubber/synthetic resin linings, and BASF suggested special copper-free steel types.
The produced hydroxylamine sulfate solution reacts with cyclohexanone at a temperature (85–90 °C) exceeding the aqueous cyclohexanone oxime melting point. The resulting aqueous ammonium sulfate phase separates from the ~7 wt% water-containing organic phase, and both are processed separately.
Cyclohexanone oxime is extracted from the ammonium sulfate phase with cyclohexanone, later recovered by distillation. Evaporative crystallization of the remaining solution yields ~0.7 kg of crystalline ammonium sulfate per kg of cyclohexanone oxime. After partial drying, the water-containing oxime is ready for Beckmann rearrangement to produce caprolactam.
1.3. Fibrant HPO® Process
Developed by Fibrant in the 1960s, the HPO (hydroxylamine phosphate oxime) process offers a unique approach to cyclohexanone oxime production based on selective nitrate ion/nitrogen oxide hydrogenation.
This combined hydroxylamine and oxime synthesis route eliminates ammonium sulfate byproduct. The HPO process enables a closed loop between hydroxylamine and oxime syntheses by avoiding sulfuric acid liberation during oxime formation,
It revolves around recycling two streams: an aqueous phosphoric acid buffer and an organic process phase. Several key reactions and operations occur within this system.
Selective catalytic hydrogenation of nitrate ions with hydrogen gas in the phosphoric acid buffer solution generates hydroxylamine. At the reaction’s pH ~2, the resulting hydroxylamine becomes protonated, forming hydroxylammonium phosphate. Pd/Pt supported on carbon or alumina is uesd as the catalyst.
A unique three-phase bubble column reactor system combines optimal gas-liquid-solid contact with efficient heat removal. Unreacted hydrogen is recycled after separation from the catalyst suspension. The hydroxylammonium phosphoric acid buffer solution proceeds to the oximation section.
The aqueous hydroxylamine-phosphoric acid buffer is reacted with an organic toluene-cyclohexanone phase converts cyclohexanone to cyclohexanone oxime and liberates phosphoric acid. This oximation step operates at pH ~2, achieving near-quantitative conversions of both hydroxylamine and cyclohexanone.
The formed organic phase (cyclohexanone oxime-toluene) is separated from the aqueous buffer, washed with water, and then toluene is distilled. Dry oxime is moved to the Beckmann rearrangement section, while distilled toluene recycles back to oximation reactor.
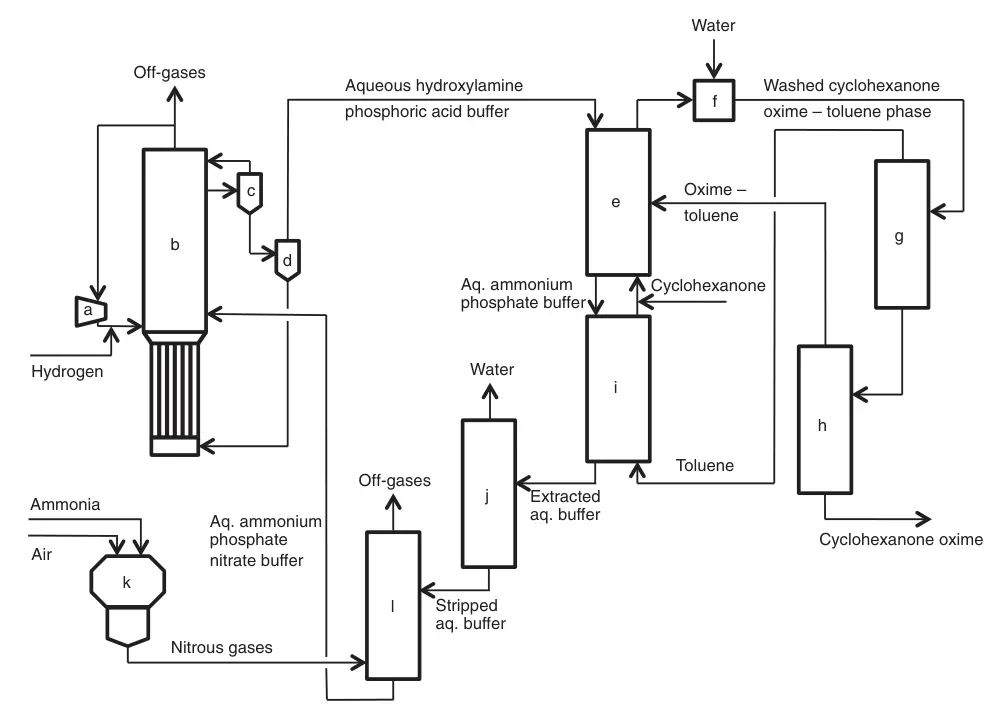
a) Compressor; b) Nitrate hydrogenation reactor; c) Gas–liquid separator; d) Catalyst filtration; e) Oximation; f) Washing; g) Toluene distillation; h) Cyclohexanone oxime purification; i) Extraction; j) Steam stripping; k) Ammonia combustion; l) Ammonia decomposition and absorption of nitrous gases
Continuous HPO process development led to the intensified HPOplus process, featuring concentrated process flows, reduced raw material/energy consumption, and significantly increased single-line oxime capacity (>200,000 t/a).
1.4. Ammoximation Process
Cyclohexanone reaction with ammonia and hydrogen peroxide offers an alternative route to cyclohexanone oxime synthesis in the liquid phase without generating ammonium sulfate byproduct.
The initial success in this approach came in 1960 with the use of tungsten and tin catalysts in aqueous ammonia at 10–30 °C, achieving high oxime yields (>90%). However, extraction and catalyst recovery challenges hindered commercialization.
In the late 1970s, a porous synthetic material (titanium silicalite (TS-1)) was developed, leading to new catalytic oxidation processes. In 1987, Montedipe implemented TS-1 for cyclohexanone ammoximation, followed by similar processes from Sinopec.
Ammonia reacts with hydrogen peroxide on the catalyst’s titanium centers, forming the intermediate hydroxylamine, which subsequently reacts with cyclohexanone to give the desired oxime.
The molar feed ratio of H2O2/cyclohexanone is 1.0-1.1 and a stable tertiary alcohol (e.g., tert-butyl alcohol) at 2-4 times cyclohexanone’s weight is used as a solvent.
The reaction is operated at a temperature of ~85 °C and a pressure of >0.25 MPa for approximatively 1.5 h to achieve a cyclohexanone to oxime conversion up to 98%.
The first commercial plant based on this technology was built by Sumitomo Chemical in 2003. Several chinese caprolactam plants use this technology, some with TS-1 variants.
A simplified process description is shown in Figure 3:
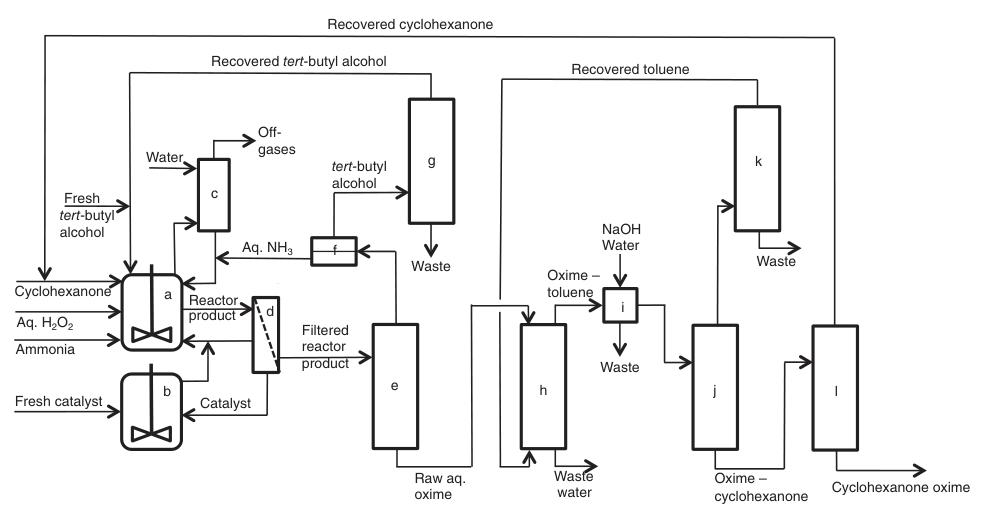
a) Ammoximation reactors; b) Catalyst preparation/purification; c) Scrubbing off-gases; d) Filtration; e) Ammonia/tert-butyl alcohol recovery; f) L/L-separation; g) tert-Butyl alcohol distillation; h) Extraction; i) Washing; j) Solvent recovery; k) Solvent distillation; l) Cyclohexanone recovery
The process begins with a continuous feedstock stream containing diluted hydrogen peroxide, cyclohexanone, ammonia, tert-butyl alcohol (both fresh and recycled), and catalyst (either fresh or recycled) entering stirred ammoximation reactors.
Off-gases generated during this step, which contain unreacted ammonia, are scrubbed with water to recover and recycle the ammonia.
The resulting product mixture then undergoes filtration and is sent to a recovery column for separation and purification. At this stage, two alternative pathways can be employed:
1. Toluene Extraction:
Unreacted ammonia and water-containing tert-butyl alcohol are recovered and purified for reuse. Cyclohexanone oxime and unreacted cyclohexanone are extracted using toluene, followed by water washing and further separation by distillation.
2. Countercurrent Re-extraction:
A continuous countercurrent re-extraction step uses demineralized water to form separate aqueous oxime and cyclohexanone phases. These phases are then subjected to further purification via distillation.
1.5. Beckmann Rearrangement of Cyclohexanone Oxime to Caprolactam
Caprolactam, a key precursor for nylon-6, is primarily produced from cyclohexanone oxime via the Beckmann rearrangement. This highly exothermic reaction occurs in both liquid and gas phases.
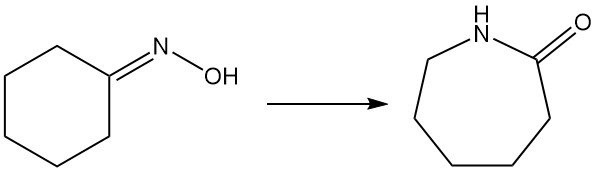
Converting oxime to caprolactam involves the traditional liquid-phase Beckmann rearrangement with fuming sulfuric acid, generating ammonium sulfate as a co-product. Sumitomo Chemical, however, uses a unique vapor-phase Beckmann rearrangement process.
A depressed ammonium sulfate price makes its co-production undesirable, motivating the development of processes minimizing or eliminating it. However, ammonium sulfate can also benefit caprolactam production when used as fertilizer in specific soil conditions, providing nitrogen, sulfur, and pH adjustment.
1.5.1. Liquid-Phase Beckmann Rearrangement
The dominant industrial method utilizes fuming sulfuric acid or oleum as a catalyst and solvent. It achieves high oxime conversion and the caprolactam yield is almost 100%, but it generates ammonium sulfate as byproduct.
The reaction parameters like temperature, acid concentration, and mixing significantly impact product purity and color. Multistage rearrangement with distributed oleum feed reduces ammonium sulfate production.
Final caprolactam purification involves solvent extraction, distillation, and washing.
1.5.2. Gas-Phase Rearrangement (Heterogeneous Catalytic Rearrangement)
The gas-phase rearrangement was developed to avoid ammonium sulfate generation associated with liquid-phase methods. Early attempts using water-eliminating catalysts struggled with low selectivity and catalyst deactivation.
Sumitomo Chemical achieved industrial success with high-silica zeolites in the presence of water and methanol.
This process involves the evaporation of a wet oxime/methanol mixture. The oxime is then converted to caprolactam over MFI zeolite catalyst at 350-380 °C. The catalyst is continuously regenerated with air at 500 °C in a fluidized-bed system.
The impurities from the resulting caprolactam are removed using distillation. Further purification of caprolactam by crystallization from a mixed solvent and via hydrogenation and multistep distillation gives a highly pure product.
Sumitomo Chemical is the only commercial producer using gas-phase rearrangement without generating ammonium sulfate.
2. Production of Caprolactam by Photooximation
In the 1950s, Toray developed a unique photochemical process to produce caprolactam. This methodology, called Photonitrosation (PNC), bypasses conventional oxime synthesis by direct conversion of cyclohexane to cyclohexanone oxime dihydrochloride, followed by subsequent Beckmann rearrangement to caprolactam.
The cyclohexane is reacted with nitrosyl chloride to give cyclohexanone oxime hydrochloride, following the reactions below:
1. Nitrosylsulfuric acid formation: Nitrous gases obtained from ammonia combustion are reacted with sulfuric acid to yield nitrosylsulfuric acid (NOHSO4).
2 H2SO4 + NO + NO2 → 2 NOHSO4 + H2O
2. NOCl production: NOHSO4 reacts with hydrogen chloride (HCl) to produce NOCl, with simultaneous regeneration of sulfuric acid.
NOHSO4 + HCl → NOCl + H2SO4
3. Photochemical Conversion:
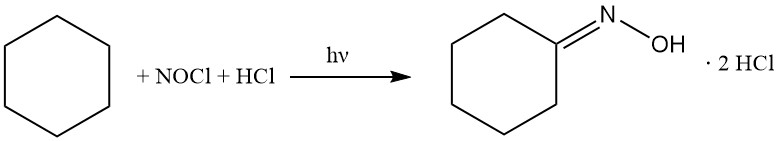
The PNC process is based on the photochemical reaction of cyclohexane with NOCl in the presence of excess HCl. This transformation is facilitated by high-performance LED lamps emitting actinic light and leads to the selective formation of cyclohexanone oxime dihydrochloride as an oily bottom layer.
Notably, LED lamps offer superior energy efficiency (0.4 kg oxime per kWh) and molar selectivity (86%) compared to earlier mercury lamp alternatives.
The recovered oxime dihydrochloride undergoes Beckmann rearrangement using oleum, yielding caprolactam. Unreacted cyclohexane is recycled, while evolved hydrogen chloride is recaptured through an aqueous absorption and concentration process.
The crude caprolactam solution is neutralized with ammonia and subjected to further purification via chemical treatment, drying, and distillation. This process generates 1.55 tons of ammonium sulfate per ton of caprolactam.
3. Recovery of Caprolactam from Nylon-6 Waste
Nylon-6 waste, generated throughout its lifecycle from production to final product disposal, presents an opportunity for resource recovery. Depolymerization processes reclaim valuable caprolactam from these waste streams, offering both environmental and economic benefits.
Solid nylon-6 waste is depolymerized to caprolactam in a high-pressure kettle reactor using superheated steam with a cracking catalyst (typically phosphoric acid). This conversion can also occur without catalyst under elevated steam pressure.
The resulting steam-caprolactam mixture undergoes partial condensation and concentration. Then, an oxidizing agent purifies the concentrate, followed by final caprolactam purification by distillation.
Washwater from nylon-6 chip production contains caprolactam oligomers alongside the monomer. Preconcentration via thin-film evaporation precedes depolymerization using the same methodology as solid waste.
The resulting aqueous caprolactam solution undergoes chemical purification and distillation to yield water, low-boiling fractions, and the desired caprolactam product.
Alternative methods also address oligomer depolymerization. One approach involves concentrating washwater to maintain oligomer solubility and feeding the caprolactam-oligomer solution into a fixed-bed or fluidized-bed reactor.
A specialized aluminum oxide catalyst cracks the oligomers at 275-350 °C, achieving a 95% yield relative to the feed. Conventional purification methods subsequently recover the caprolactam.
Several established technologies deal with postconsumer nylon-6 waste, particularly discarded carpets. These processes necessitate collection, sorting, and mechanical shredding before depolymerization to virgin-grade caprolactam.
Notable examples include “6ix Again” program by BASF, which uses a semicontinuous reactor with phosphoric acid and superheated steam for high-temperature (250-300 °C) depolymerization. Subsequent purification with an oxidizing agent and vacuum distillation recovers caprolactam from the distillate.
Other process is Evergreen Nylon Recycling which is a two-stage non-catalyzed depolymerization method that employs a high-pressure reactor system and superheated steam to hydrolyze shredded material. It recovers 90-95% of the initial nylon-6 as caprolactam, suitable for carpet production.
4. Toluene-based Process
Developed by Snia Viscosa in 1960 and used in Italy until the early 1990s, this toluene-based process offers an alternative route to caprolactam production. The process encompasses three key steps:
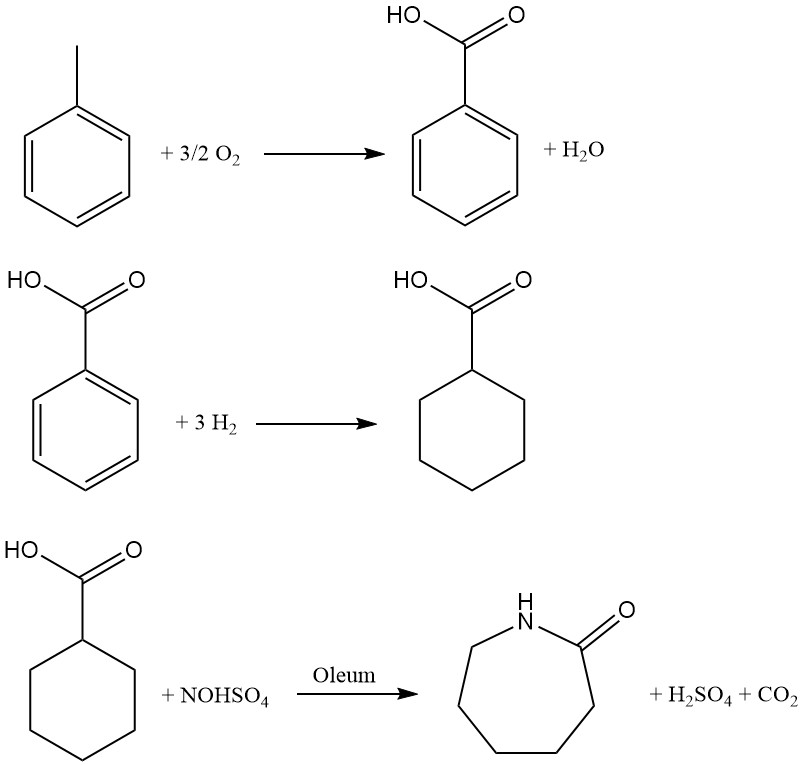
1. Catalytic oxidation: Toluene undergoes air oxidation in the liquid phase using a cobalt catalyst at 160-170 °C and 0.8-1 MPa pressure. Recovering unreacted toluene via cooling ensures optimal yield. The resulting liquid product, containing roughly 30% benzoic acid alongside intermediates and byproducts, necessitates multistage distillation for purification.
2. Hydrogenation: Benzoic acid is converted to cyclohexanecarboxylic acid through hydrogenation in the liquid phase using a Pd/C catalyst. A series of stirred reactors operating at approximately 170 °C and 1-1.7 MPa pressure facilitate efficient transformation. Subsequent distillation under reduced pressure purifies the resulting acid.
3. Nitrosodecarboxylation and rearrangement: This crucial step combines nitrosation of cyclohexanecarboxylic acid with Beckmann rearrangement to directly generate caprolactam. A 73% nitrosylsulfuric acid solution in sulfuric acid, prepared by conventional NO absorption in oleum, is used in the reaction within a multistage reactor.
Maintaining the conversion rate around 50% requires the process to occur in boiling cyclohexane under atmospheric pressure. Sulfonic acids form as inevitable byproducts.
Following the combined nitrosation-rearrangement, the product stream undergoes hydrolysis with water at low temperatures. Extracting unreacted cyclohexanecarboxylic acid allows for its recycling back into the process.
The acidic caprolactam solution, containing excess sulfuric acid, then moves to the neutralization stage. Crystallization under reduced pressure with ammonia yields two distinct liquid layers: an ammonium sulfate solution and an aqueous caprolactam solution. Further purification involves:
- Toluene extraction separates caprolactam from water-soluble byproducts.
- Countercurrent extraction with water recovers caprolactam from the toluene solution.
- Distillation yields pure caprolactam from the concentrated aqueous solution.
5. Alternative Processes
Beyond the conventional cyclohexanone ammoximation process, several alternative routes to caprolactam have emerged:
5.1. Hydrogen Peroxide Processes
- Inventa process: Cyclohexylamine oxidation with hydrogen peroxide generates cyclohexanone oxime.
- 1,1´-Peroxydicyclohexylamine process: Cyclohexanone and hydrogen peroxide react to form a peroxide, which then converts to caprolactam and regenerates cyclohexanone.
5.2. Oxidation of Cyclohexylamine with Elemental Oxygen:
Direct oxidation of cyclohexylamine with oxygen produces cyclohexanone oxime.
5.3. Direct Oximation of Cyclohexanone:
Ammonia, air, and cyclohexanone directly react over various catalysts to form cyclohexanone oxime.
5.4. Bis(nitrosocyclohexane) Process:
Electric discharge triggers the reaction of cyclohexane with nitric oxide or related compounds, forming bis(nitrosocyclohexane), which rearranges to cyclohexanone oxime.
5.5. ε-Hydroxycaproic Acid/ε-Caprolactone Process:
Oxidation of cyclohexanone with air generates ε-hydroxycaproic acid and adipic acid (byproduct). Both can be converted to caprolactam via different routes.
5.6. 2-Nitrocyclohexanone Process:
A unique sequence of reactions involving acetylation, nitration, ring cleavage, hydrogenation, and cyclization converts cyclohexanone to caprolactam without byproducts.
5.7. 3,3-Pentamethyleneoxaziridine Process:
Hypochlorite reacts with cyclohexanone in aqueous ammonia to form cyclohexanone isoxime, which then rearranges to caprolactam under heat.
5.8. Catalytic Deacetylation of N-Acetylcaprolactam:
O-acetylcyclohexanone oxime undergoes deacetylation over catalysts to yield caprolactam.
5.9. Butadiene-Based Processes:
- ALTAM process: Developed by DSM and others, this multi-step process utilizes butadiene to ultimately produce caprolactam with high selectivity.
- Adiponitrile-based processes: BASF and Rhone-Poulenc developed independent processes for caprolactam production from adiponitrile, obtained from various routes including butadiene.
5.10. Biorrenewable Routes:
With increasing sustainability concerns, bio-based resources are gaining traction as feedstocks for caprolactam production. Several promising routes are under development, focusing on:
- Drop-in chemicals: Bio-derived benzene, phenol, acrylonitrile, or HMF can be readily integrated into existing caprolactam processes.
- Chemical routes: Levulinic acid or furfural, obtained from biomass, can be converted to intermediates leading to caprolactam.
- (Partially) fermentative routes: Microbial synthesis of lysine or direct fermentation of carbohydrates offer potential routes to caprolactam precursors.
While currently not economically competitive with traditional methods, biorenewable processes hold promise for future sustainable caprolactam production.
Reference
- Caprolactam; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a05_031.pub3
