Cyanuric Acid: Properties, Reactions, Production and Uses
What is Cyanuric acid?
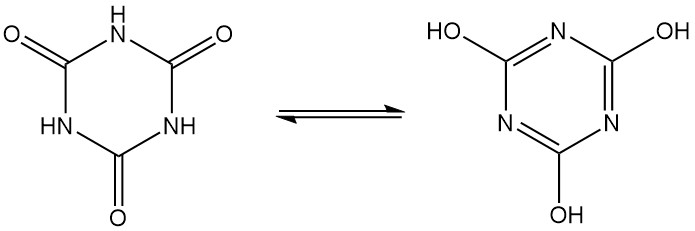
Cyanuric acid, also known as 1,3,5-triazine-2,4,6(1H,3H,5H)-trione, is an odorless white crystalline solid with a molecular formula of C3H3N3O3. It is a cyclic trimer that interconverts between several structures via lactam-lactim tautomerism.
Although cyanuric acid has been known since 1776, it only gained industrial importance in the 1950s.
Table of Contents
1. Physical Properties of Cyanuric Acid
Cyanuric acid is a white crystalline solid that forms a colorless crystalline dihydrate from water and loses water upon exposure to dry air. It is a weak tribasic acid that is slightly soluble in water and organic solvents like acetone, benzene, ether, ethanol, hexane, and isopropyl alcohol.
The following table shows the solubility of cyanuric acid in various solvents at 25 °C (wt%).
| Solvent | Solubility (wt%) |
|---|---|
| Dimethyl sulfoxide | 17.4 |
| Sulfuric acid (98 wt%) | 14.1 |
| Dimethylformamide | 7.2 |
| N-methyl-2-pyrrolidone | 6.3 |
| Dimethylacetamide | 3.0 |
| Pyridine | 2.2 |
The important physical properties of cyanuric acid are listed in Table 1.
| Property | Value |
|---|---|
| CAS number | 108-80-5 |
| Formula | C3H3N3O3 |
| Molecular weight | 129.07 g/mol |
| Melting point | 320 – 330 °C (decomposition) |
| Density | 1.80 g/cm³ |
| pKa1 | 6.88 |
| pKa2 | 11.40 |
| pKa3 | 13.5 |
2. Reactions of Cyanuric Acid
Cyanuric acid reacts with inorganic and organic bases to form salts, which favor the hydroxytautomer in alkaline solutions.
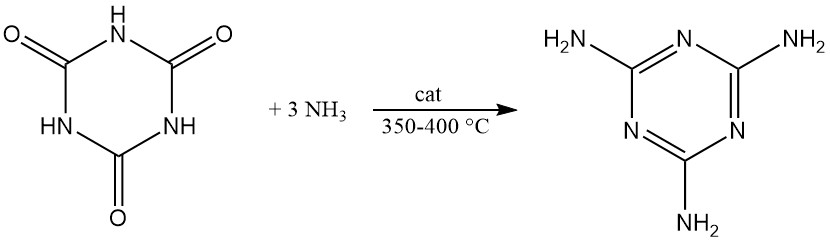
Under pressure and with a catalyst at 350–400 °C, cyanuric acid reacts with ammonia to produce melamine.
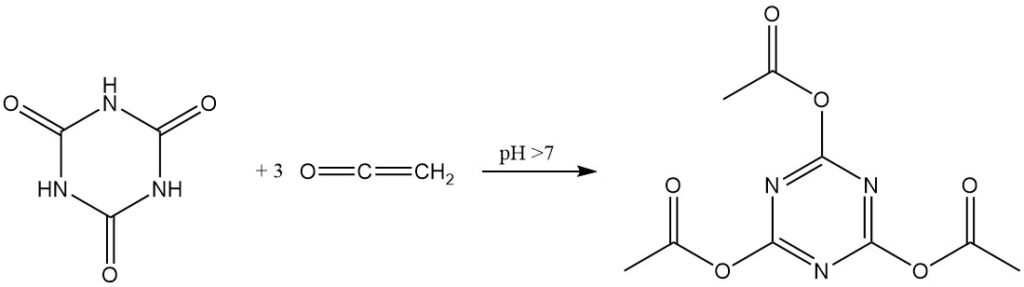
In the presence of alkaline catalysts, ketene reacts with cyanuric acid to form triacetyl cyanurate in high yield.
Cyanuric acid reacts primarily as a cyclic imide. The nucleophilic isocyanurate nitrogen atom, often in anionic form, attacks a positively polarized carbon atom. This typically leads to trisubstituted products.
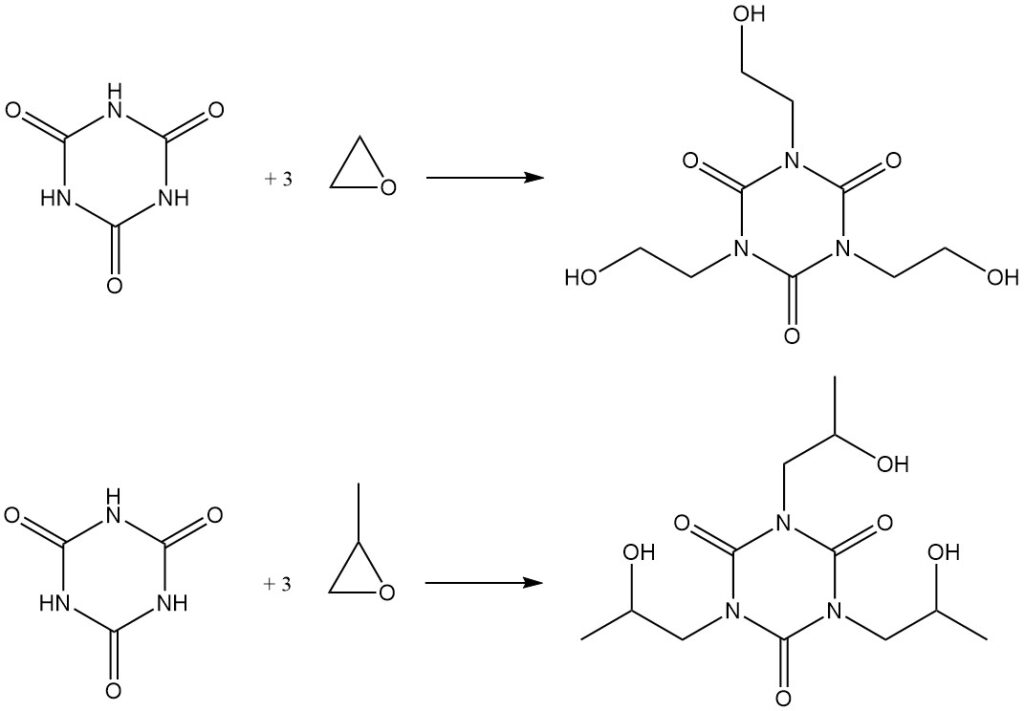
Heating cyanuric acid with epoxides in DMF results in the formation of hydroxyalkyl isocyanurates. Examples include the synthesis of tris (2-hydroxyethyl) isocyanurate from ethylene oxide and the corresponding hydroxypropyl derivative from propylene oxide.
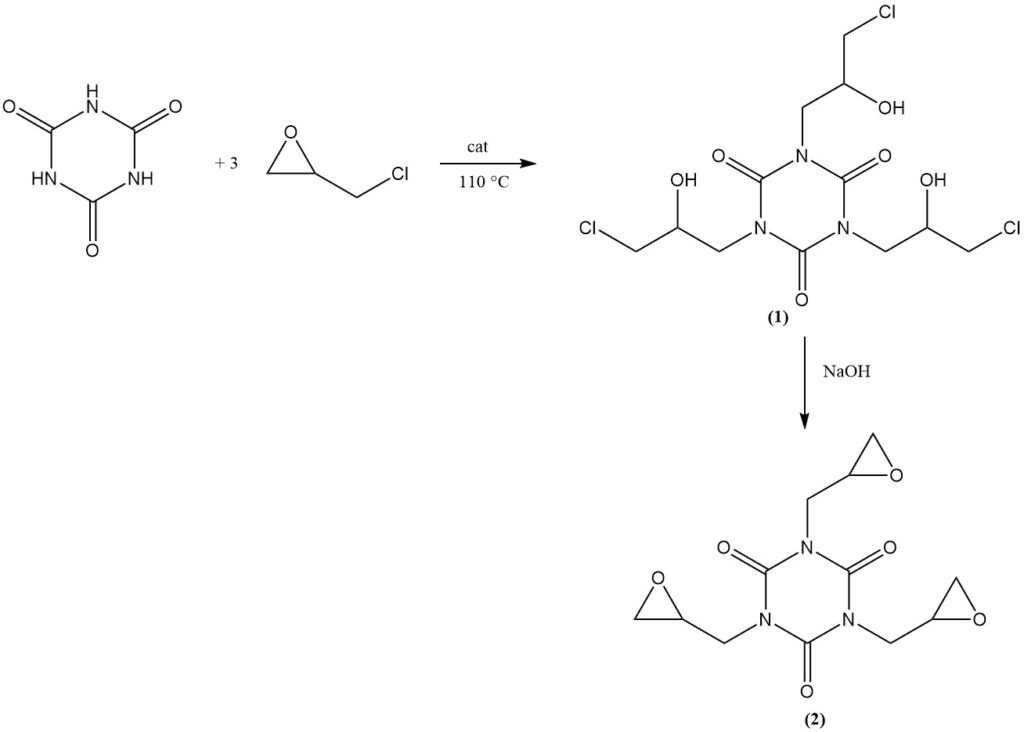
The reaction of cyanuric acid with epichlorohydrin in the presence of a basic catalyst at 110 °C yields tris (3-chloro-2-hydroxypropyl) isocyanurate (1). Subsequent dehydrochlorination in an aqueous alkaline solution produces the commercial product tris(2,3-epoxypropyl) isocyanurate (2).
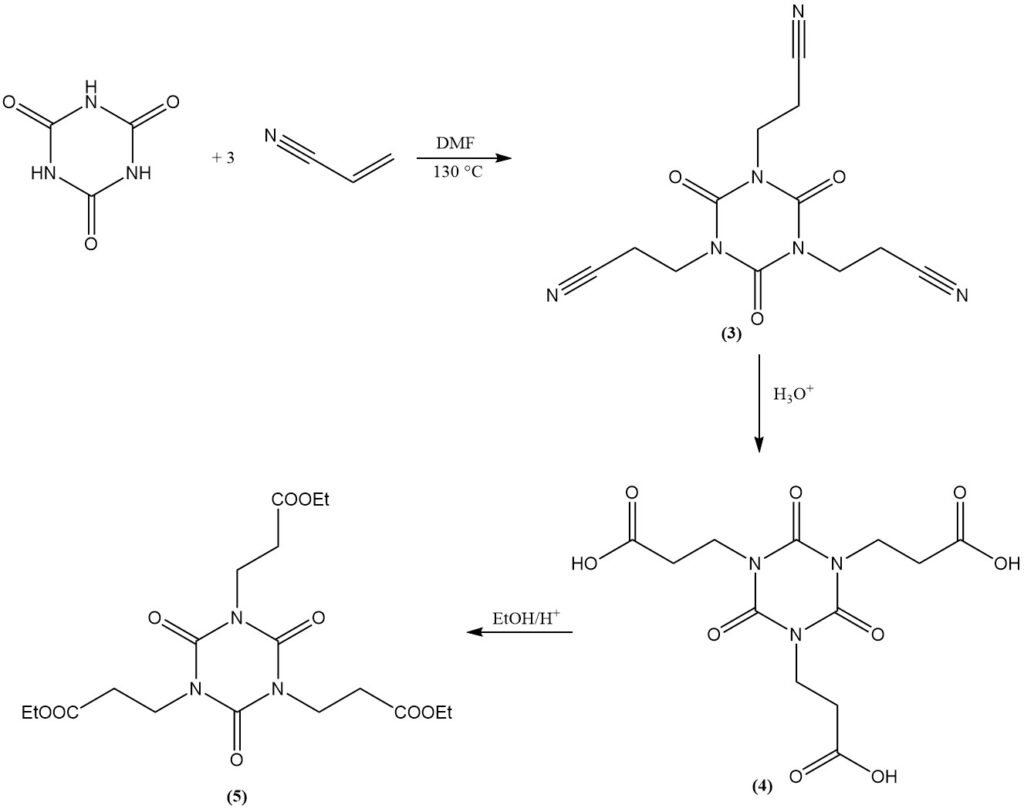
Cyanuric acid undergoes addition reactions with double bonds. An example is the formation of bis(2-cyanoethyl) and tris(2-cyanoethyl) isocyanurates from acrylonitrile in DMF at 130 °C. Tris(2-cyanoethyl) isocyanurate (3) can be saponified to yield tris(2-carboxyethyl) isocyanurate (4).
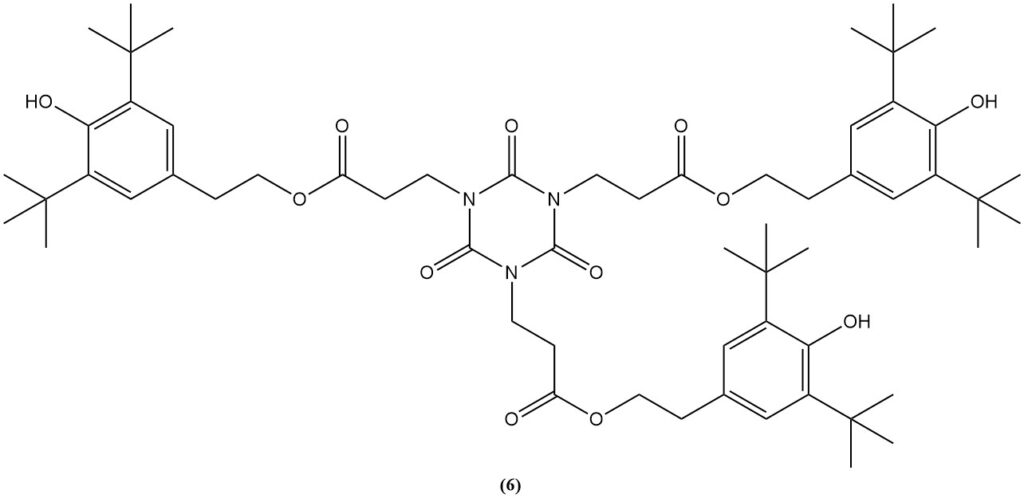
The esterification of the carboxyethyl derivative with ethanol produces tris(2-carbethoxyethyl) isocyanurate (5), a precursor for the commercial light stabilizer and antioxidant (6).
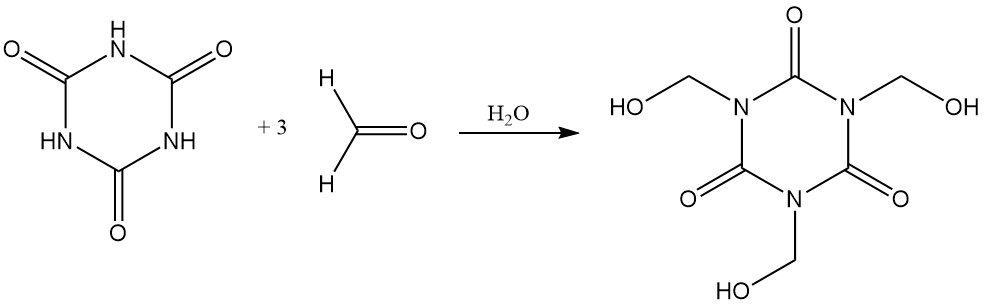
Cyanuric acid readily dissolves in aqueous formaldehyde to form tris (hydroxymethyl) isocyanurate.
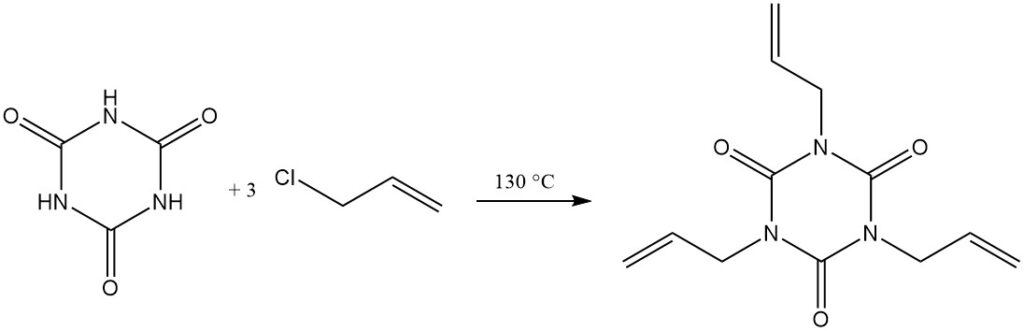
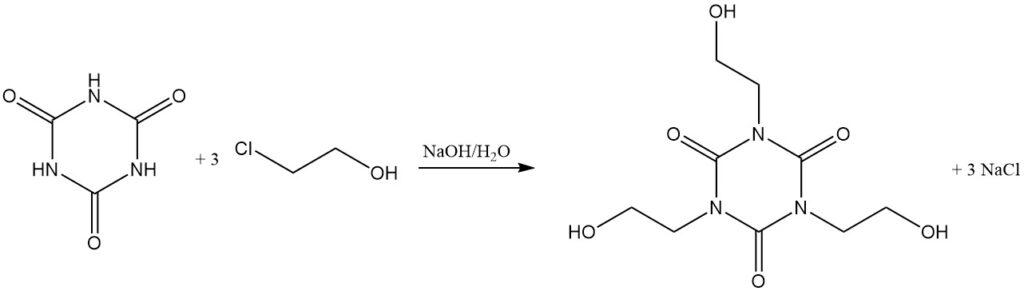
At elevated temperatures and with a proton acceptor, alkyl halides react with cyanuric acid. Examples include the reaction with allyl chloride in dichlorobenzene/triethylamine at 130 °C to form triallyl isocyanurate and with 2-chloroethanol in aqueous sodium hydroxide to form tris (2-hydroxyethyl) isocyanurate.
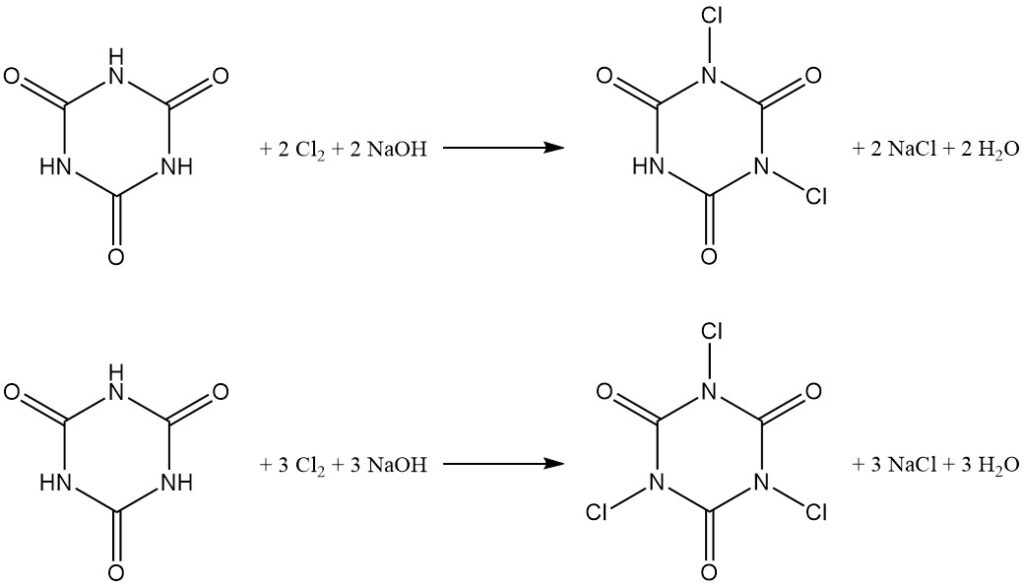
Controlled-pH chlorination of cyanuric acid in water yields N,N’-dichloro and N,N’,N”-trichloro derivatives (see the article about Chloroamines).
The product distribution depends on the sodium hydroxide to cyanuric acid molar ratio, with a 2:1 ratio favoring N,N’-dichloroisocyanuric acid (DCCA) and a 3:1 ratio favoring N,N’,N”-trichloroisocyanuric acid (TCCA) at a 90% yield.
Above 200 °C, cyanuric acid decomposes slowly, accelerating at its melting point (320–330 °C). The primary product is isocyanic acid. At higher temperatures, decomposition reactions likely involve ring cleavage via a cyanic acid intermediate.
3. Production of Cyanuric Acid
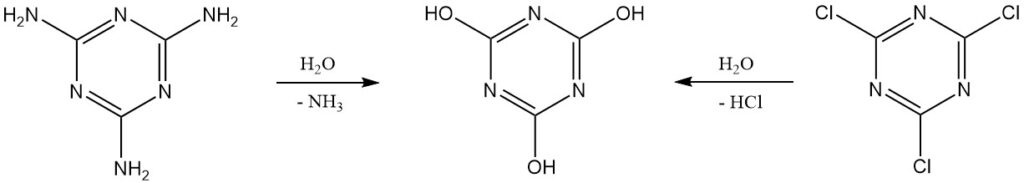
Pure cyanuric acid can be produced in the lab by the hydrolysis of cyanuric chloride or melamine. Further purification involves recrystallization from dimethylformamide or using sodium or ammonium salts.
Cyanuric acid is manufactured commercially by the thermal decomposition (pyrolysis) of urea at 200–300 °C, which generates ammonia as a byproduct.
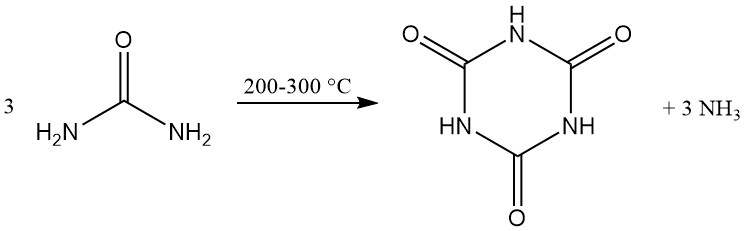
Numerous patented methods focus on improving heat and mass transfer by designing special reactors or modifying reaction mixtures. Reactors used include heated screws, rotating drums, conveyor belts, rotary-tube furnaces, and ball mills.
Fluidized beds with AlF3 particles or cyanuric acid crystals and high-frequency induction heating of molten urea have also been explored.
The caking problem during the process is solved by using premixed powders with specific urea to cyanuric acid ratios, preformed urea cyanurate, or by recycling the crude product.
Molten metals or salts can be used as heat transfer mediums instead of direct heating. High-boiling solvents like polyglycol ethers and sulfolanes that dissolve urea but not cyanuric acid can be used to avoid localized overheating and minimize isocyanic acid formation.
Crude cyanuric acid typically contains up to 30% impurities like melamine and its precursors. Purification methods include acid treatment and solubility-based separation.
Dilute mineral acids convert impurities to ammonia, carbon dioxide, or cyanuric acid itself. Another method is that the crude acid is dissolved in an alkaline solution (NaOH or dilute ammonia), and then the organic impurities are filtered. The cyanuric acid is then precipitated by adding mineral acids.
4. Uses of Cyanuric Acid
The most important use of cyanuric acid is in the production of N-chlorinated isocyanurates. These chlorine-containing derivatives are widely used as disinfectants and sanitizing agents in:
- Swimming pool disinfectants
- Household bleaches
- Industrial and institutional cleaners
- Dishwasher detergents
Cyanuric acid is also used to help stabilize chlorine in swimming pool water by reducing its deactivation rate by a factor of 5–10 when used at concentrations of 25–50 ppm.
It is approved for use as a source of nitrogen in ruminant feed. Cyanuric acid is a precursor of isocyanic acid.
Small quantities of cyanuric acid are used to reduce NOx emissions in exhaust gases from stationary diesel engines and boilers fueled by coal, oil, or gas.
Several isocyanurate derivatives of cyanuric acid are used in the plastics industry. Examples include:
- Triallyl isocyanurate: homopolymerized or co-polymerized, it functions as a cross-linking agent in polyethylene, polyvinyl chloride (PVC), and laminate formulations.
- Tris(2-hydroxyethyl) isocyanurate: This compound acts as a cross-linking agent for polyurethanes, polyesters, and alkyd resins used in wire enamels and electrical varnishes.
- Triglycidyl isocyanurate: It is a cross-linking agent in epoxy resins and a curing agent for weather-resistant powder coatings.
Melamine cyanurate has gained commercial importance as a halogen-free flame retardant for polyamide resins. It is also used as a potential solid lubricant.
Tris(2-carboxyethyl) isocyanurate is used in the production of water-soluble alkyd resins. Its ester derivatives serve as plasticizers for PVC and lubricants.
5. Toxicology of Cyanuric Acid
Cyanuric acid is generally considered a low-hazard product. It is classified as essentially nontoxic (acute oral and dermal) and non-irritating to the skin and eyes.
Acute Toxicity Data:
- LD50 (rat, oral) > 5000 mg/kg (Practically non-toxic)
- LD50 (rabbit, dermal) > 5000 mg/kg (Practically non-toxic)
- LC50 (fish) > 2000 mg/L (24h) (Low toxicity)
- EC50 (Daphnia) > 2000 mg/L (Low toxicity)
Subacute Toxicity Data:
- NOEL (fish): 1000 mg/L
- NOEL (algae): 2500 mg/L
- Inhibition of reproduction (Daphnia): 1000 mg/L
Even though cyanuric acid has low inherent toxicity, inhalation of dust, eye contact, and ingestion should still be avoided. Be aware that it decomposes to toxic isocyanic acid above 200 °C.
Reference
- Cyanuric Acid and Cyanuric Chloride; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a08_191
