
Table of Contents
1. Physical Properties of 2-Chloroethanol
Dilute solutions of 2-chloroethanol in water have a somewhat sweet, pleasant odor. It is a flammable liquid that can form an azeotrope with water that boils at 97.8 with 42% H2O wt. Ethylene chlorohydrin is miscible with water, ethanol, acetone, and benzene. The general physical properties of 2-chloroethanol are listed in Table 1.| Property | Value |
|---|---|
| CAS Registry Number | [107-07-3] |
| Molecular Weight | 80.52 g/mol |
| Melting Point (mp) | -69 °C |
| Boiling Point (bp) | 129 °C |
| Density at 20 °C | 1.2133 g/mL |
| Refractive Index | 1.44 |
| Viscosity (η) | 3.43 mPa.s |
| Vapor Pressure | 700 Pa (at 20 °C) |
| Flash Point | 57 °C |
| Autoignition Temperature | 425 °C |
| Explosive Limit | 5–16% |
2. Chemical Reactions of 2-Chloroethanol
2-Chloroethanol undergoes reactions that are characteristic of both alcohols and alkyl chlorides. The most common reaction of 2-chloroethanol is dehydrochlorination to form ethylene oxide, which was used industrially but became less important because of alternative methods.
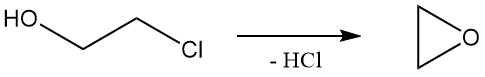
The relative rate of solvolysis of ethylene chlorohydrin in water at 97 °C is 1.0.
Hydroxyethyl ethers are produced by reacting 2-chloroethanol with alcohols or phenols under basic conditions. Hydroxyethyl cellulose and modified starch are manufactured by treating these materials with ethylene chlorohydrin.
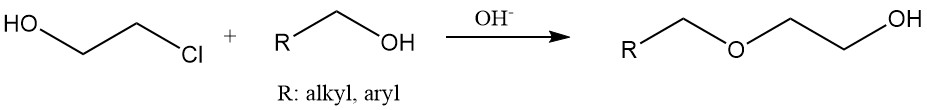
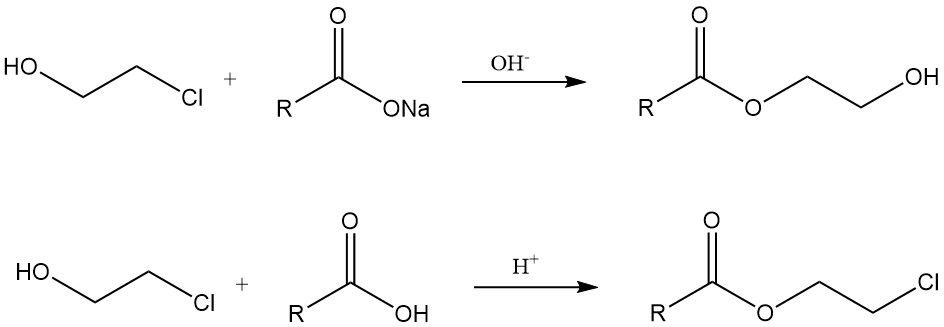
Chlorohydrins react with metal carboxylates in the presence of a base to give hydroxyethyl esters. On the other hand, β-chloro esters are formed by reactions with carboxylic acids under acidic conditions or from acid chlorides.
Cyclic carbonate may be produced by the reaction of 2-chloroethanol with carbon dioxide in the presence of an amine.
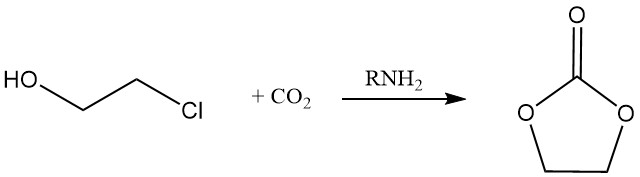
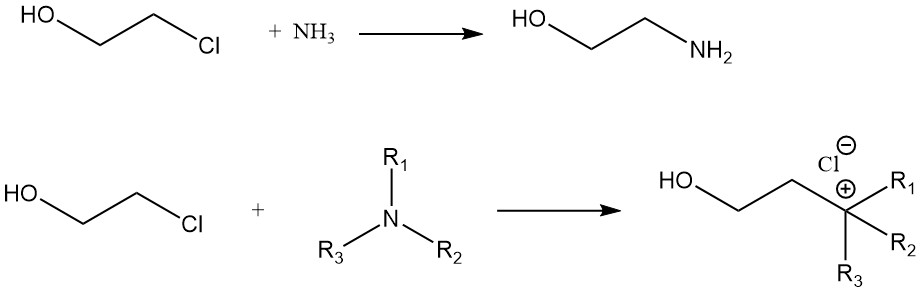
A comparison of the rate of reaction of ethylene chlorohydrin with various amines gives the following order: n-amylamine > cyclohexylamine > aniline. The reaction of ethylene chlorohydrin with ammonia gives monoethanolamine. Quaternary ammonium compounds are formed from ethylene chlorohydrin and tertiary amines.
Other 2-chloroethanol reactions include the formation of nitriles from cyanides, acetals from aldehydes, oxazolidinones from cyanates, and the oxidation of ethylene chlorohydrin to monochloroacetic acid.
The reaction of 2-chloroethanol with phosgene forms 2-chloroethyl chloroformate.
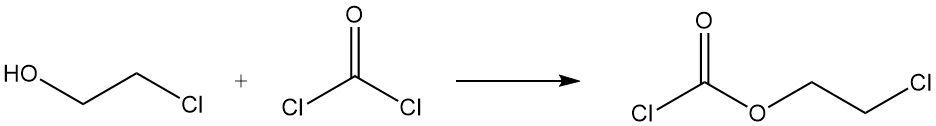
3. Production of 2-Chloroethanol
As early as 1904, BASF produced 2-chloroethanol by reacting ethylene and CO2 in an aqueous bleaching powder solution.
Today, 2-chloroethanol is produced by the hypochlorination of ethylene with hypochlorous acid, which is formed by the reversible reaction of chlorine and water, resulting in limited efficiency.
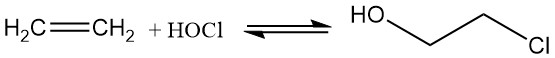
Cl2 + H2O ⇌ HOCl + HCl ; K = 4.2 x 10-4
The reaction between ethylene and hypochlorous acid is faster than the reaction of ethylene and chlorine to form dichloroethane, so as the ethylene is added to the aqueous system, chlorohydrin is produced preferentially.
A minimal quantity of dichloroethane is formed with good stirring in the gas phase if the 2-chloroethanol concentration doesn’t exceed 6–8%. However, increasing HCl concentration reduces available HOCl, leading to significant dichloroethane formation after HCl is greater than 3%.
Due to dichloroethane’s low water solubility (0.869 g/100 mL at 20°C), it forms a separate phase, dissolving both chlorine and ethylene for further dichloroethane formation.
Laboratory Studies and Reactor Optimization
A lab study investigated hypochlorination parameters using single-column and recycle-reactor setups. The results show that temperatures of 35–50 °C and a 50% ethylene excess were preferred for efficient dichloroethane stripping.
A 88% yield of 2-chloroethanol was achieved using continuous operation at 6.4% ethylene chlorohydrin concentration, a temperature of 35 °C, a 1.42 ethylene:chlorine ratio, and 71 g/h chlorine feed.
2-Chloroethanol production for ethylene oxide has been replaced by direct ethylene oxidation with silver catalysts. However, propylene oxide and epichlorohydrin are still made by the direct oxidation of the parent olefin.
In the industrial-scale production of ethylene chlorohydrin, chlorine, ethylene, and water were co-introduced upwards through packed towers. These reactors minimize ethylene-chlorine contact and maximize hydrocarbon-liquid phase contact.
The feed lines are strategically positioned to ensure complete chlorine dissolution before ethylene introduction. Some plants use separate mixing columns for chlorine and water before the ethylene reaction tower.
The resulted product stream contains a 4.5–5.0% 2-chloroethanol solution with yields reaching 85-89% of converted ethylene. Dichloroethane and bis(2-chloroethyl) ether are minor byproducts.
4. Uses of 2-Chloroethanol
2-Chloroethanol was primarily used for the synthesis of ethylene oxide, but it still finds diverse applications across various industries.
2-Chloroethanol is used as a catalyst for olefin metabolism and cycloalkenering-opening polymerization in the form of adducts with tungsten or molybdenum halides and organoaluminum compounds.
It is used in producing dyes, pharmaceuticals, biocides, plasticizers, and thiodiglycol.
It is used as a raw material in the production of modified cellulose (hydroxyethyl cellulose) and strach, and to dissolve various materials, including cellulose acetate and ethyl cellulose used in coatings and films. It finds application in textile printing for dissolving dyes and in processes like dewaxing oils, refining rosin, extracting pine lignin, and even cleaning machinery.
5. Toxicology of 2-Chloroethanol
Ethylene chlorohydrin poses significant health risks. Safe handling requires skin, eye, and respiratory protection due to its high dermal toxicity and moderate oral and inhalation toxicity.
2-chloroethanol can be absorbed through skin, inhalation, and ingestion. Acute exposures can be fatal via skin contact or inhalation. Symptoms include eye damage, respiratory distress, gastrointestinal distress, and organ damage.
Toxicity data:
- Oral: moderately toxic, with LD50 values around 60–95 mg/kg in rats.
- Skin: can be absorbed through the skin, potentially reaching harmful levels. Local irritation is minimal.
- Eye: Contact can cause serious damage or even blindness. Immediate flushing is crucial.
- Inhalation is fatal at saturated vapor concentrations. The estimated LC50 is between 16 and 62 ppm.
- There is no evidence of reproductive or developmental effects in animal studies.
- Mutagenicity: Positive and negative results in various tests suggest the potential for base-pair substitution mutations.
- Carcinogenicity: There is no evidence of carcinogenicity in animal studies. Human data is inconclusive.
2-Chloroethanol is classified as flammable, acutely toxic by various routes, and causes serious eye damage. Occupational exposure limits vary by agency, ranging from 1–5 ppm.
Reference
- Chlorohydrins; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_565.pub2


