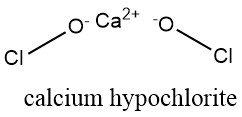
Solid hypochlorite is a powder that is white, soft, and dry. It is virtually odorless, or it may smell more or less strongly of chlorine or hydrochloric acid due to decomposition during storage.
The stability of hypochlorite is mainly dependent on its water content, which is usually less than 1%.
Tropical bleach contains even less than 0.3%. They remain stable up to 80°C, while tropical bleach remains stable even up to 100°C. When heated to 180°C, they decompose into chloride and oxygen.
The stability of hypochlorites is decreased by metals like iron, nickel, or cobalt. As a result, the raw materials used for hypochlorite production must be free of these metals.
Properly stored tropical bleach (< 0.3% water) that is free of heavy metals has a shelf life of over two years.
Table of Contents
1. Production of calcium hypochlorite
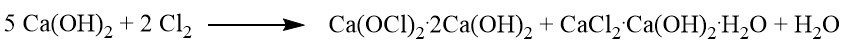
1.1 Standard Bleaching Powder
The standard bleaching powder is a mixture comprising calcium hypochlorite (Ca(OCl)2), calcium chloride, calcium hydroxide, and varying amounts of water. The production process involves the passage of chlorine gas over hydrated lime.
The Dynamit Nobel’s Rheinfelden bleaching powder manufacturing process is conducted as a batch operation. Dry powdered lime hydrate is chlorinated in a horizontal reaction drum at 45 °C and low pressure (5.3 kPa), with chlorine being injected in liquid form. The reaction mixture is permanently mixed using a slowly rotating rake.
The reaction between the solid lime hydrate and chlorine results in the formation of a mixture of dibasic calcium hypochlorite (Ca(OCl)2·2 Ca(OH)2) and basic calcium chloride, which corresponds to 40% of the available calcium hydroxide.
Further chlorination leads to the formation of hemibasic calcium hypochlorite (Ca(OCl)2· 1/2 Ca(OH)2) and neutral calcium chloride hydrate. The bleaching powder reaction stops after approximately 60% of the available calcium hydroxide is converted.
The exothermic reaction generates 1100 kJ of heat per kg of chlorine converted, which together with the low pressure causes water and liquid chlorine to evaporate.
Consequently, the reaction mixture is dried entirely under vacuum at a maximum temperature of 85 °C, yielding standard bleaching powder containing 35–37 % available chlorine content.
Alternatively, gaseous chlorine can be used for the reaction, although the reaction rate is slower, chlorine losses are higher, and the available chlorine content of the product is smaller.
1.2 Tropical Bleach
Finely ground quicklime (CaO) is added to standard bleaching powder to reduce water content further. The quicklime absorbs any residual water present and is converted into calcium hydroxide.
Although this operation decreases the available chlorine content by 1–2%, the additional drying yields tropical bleach, which is stable up to temperatures of 100 °C.
ICI developed a continuous process for the production of bleaching powder where countercurrents of calcium hydroxide and chlorine react in a rotating drum.
The heat generated during the reaction is removed by spraying the drum externally with water, and the chlorine is diluted with cooled air. This gas stream also removes the water formed during the chemical reaction.
1.3 High-Percentage Hypochlorite
Chlorinating slurries of calcium compounds such as calcium hydroxide or bleaching powder yields solid hypochlorites with 70% or higher available chlorine contents. The initial product formed is hemi-basic calcium hypochlorite (Ca(OCl)2· 1/2 Ca(OH)2), which upon further chlorination gives neutral calcium hypochlorite dihydrate (Ca(OCl)2·2 H2O).
The neutral calcium hypochlorite dihydrate is then dried to the desired high-percentage hypochlorite.
In all of these reactions, calcium chloride is formed as a byproduct. Some processes recover the calcium values by adding sodium hypochlorite to the slurries, resulting in a product that primarily consists of calcium hypochlorite, sodium chloride, and water, which is then removed.
2. Uses of bleaching powder
In the paper manufacturing, calcium hypochlorite finds use in the single-stage bleaching process.
On the other hand, the multistage process, which involves chlorination, caustic extraction, and hypochlorite oxidation, employs the more costly sodium hypochlorite.
When sodium hypochlorite is used instead of calcium hypochlorite for the bleaching of Kraft pulp, higher brightness and greater strength are achieved.
In laundry operations, bleaching powder is first suspended in water and then decanted. Only the solution is utilized since the insoluble matter may inflict damage on the fibers.
Reference
- Chlorine Oxides and Chlorine Oxygen Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_483.pub2