Propylene chlorohydrin is a general term that refers to the two isomers of chloropropanol (1-chloro-2-propanol and 2-chloro-1-propanol) with the chemical formula C3H7ClO. It is a colorless liquid with a pleasant odor, and it is soluble in water and organic solvents.
- 1-Chloropropanol is the more common isomer, and it is the one that is typically referred to when someone says “propylene chlorohydrin.”
- 2-Chloropropanol is less common, and it is not as commercially important as propylene chlorohydrin.
Table of Contents
| Property | 2-Chloro-1-propanol | 1-Chloro-2-propanol |
|---|---|---|
| CAS Number | [78-89-7] | [127-00-4] |
| Formula | CH3CHClCH2OH | CH3CHOHCH2Cl |
| Molecular Weight (g/mol) | 94.54 | 94.54 |
| Form | Colorless liquid | Colorless liquid |
| Boiling Point (°C) | 133 | 127 |
| Flash Point (°C) | 52 | 52 |
| Density at 20°C (g/ml) | 1.103 | 1.115 |
| Viscosity (mP.s) | - | 4.67 |
| Vapor Density | - | 3.3 |
| Vapor Pressure at 20°C (mmHg) | - | 4.9 |
| Solubility | Very soluble in water, soluble in organic solvents | Miscible with water and organic solvents |
| Refractive Index at 20°C | 1.4362 | 1.4392 |
2. Chemical Reactions of Propylene Chlorohydrin
Propylene chlorohydrin, like 2-chloroethanol, reacts as both alcohols and alkyl chlorides. It participates in several important chemical reactions. Here are some key reactions:
Dehydrochlorination: The most important reaction of propylene chlorohydrin is dehydrochlorination using basic catalysts such as NaOH or Ca(OH)2 to yield propylene oxide. This oxide is an important precursor for producing propylene glycol.
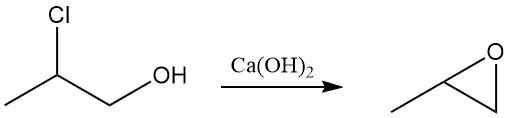
Ether Formation: Under basic conditions, propylene chlorohydrin readily reacts with alcohols or phenols to produce hydroxypropyl ethers. This reaction is used in the industrial synthesis of cellulose and starch ethers.
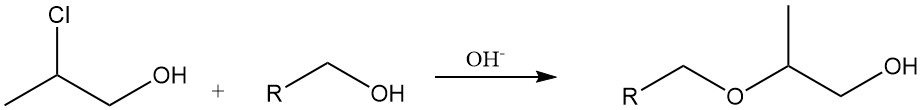
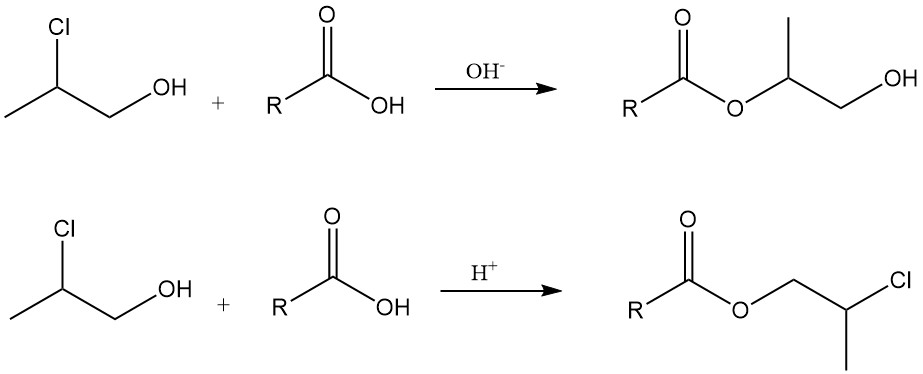
Esterification: propylene chlorohydrin reacts with carboxylic acids and their derivatives. In the presence of a base, they form hydroxyalkyl esters, while in acidic conditions or by reactions with acid chlorides, they produce β-chloro esters.
4-Methyl-1,3-dioxolan-2-one (a cyclic carbonate) can be obtained by treating propylene chlorohydrin with carbon dioxide in the presence of an amine.
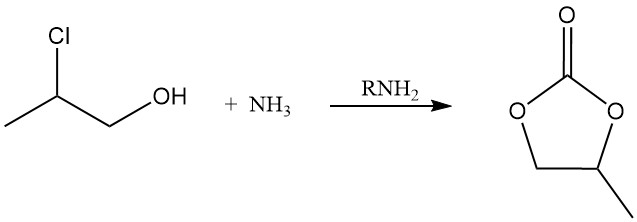
Hydrolysis: Under acidic conditions, propylene chlorohydrin undergoes hydrolysis, where the chlorine atom is replaced by a hydroxyl group, forming propylene glycol.
Reactions with amines: propylene chlorohydrin reacts with ammonia to give 2-aminopropanol and with tertiary amines to yield quaternary ammonium compounds.
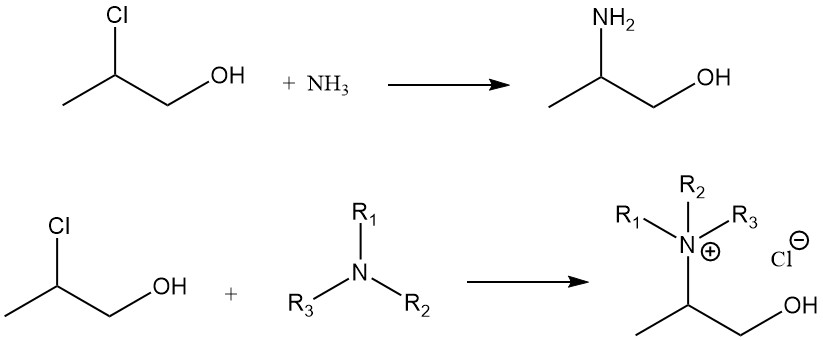
Chlorination: The hydroxyl group of propylene chlorohydrin, with specific chlorinating agents such as SOCl2, PCl3, or PCl5, can be substituted with a chlorine atom to form 1,2-dichloropropane.
Other Reactions: Propylene chlorohydrin can also participate in oxidation and other nucleophilic substitution reactions, such as the production of nitrile from cyanides, acetal from aldehydes, and oxazolidinone from cyanates.
3. Production of Propylene Chlorohydrin
Propylene chlorohydrin is produced by the controlled hypochlorination of propene. This process yields two isomers, with 1-chloropropan-2-ol (90%) as the main product and its isomer 2-chloropropan-1-ol (10%) as a minor product. 1,2-dichloropropane and bis(2-chloro-1-methylethyl) ether are formed as unwanted byproducts.
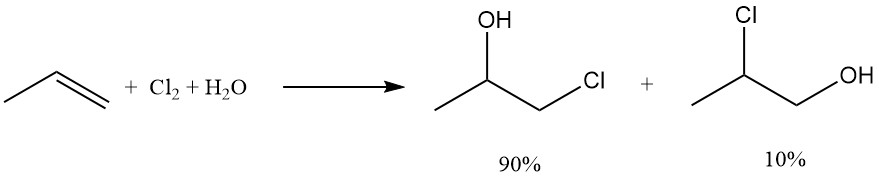
In the propylene chlorohydrin process, it is critical to separate the feed for chlorine and propene to avoid the formation of dichloropropane as the main product.
A recirculating-type, double-chamber reaction system was used to achieve propylene chlorohydrin yields of 87.5% with minimal byproducts. In comparison, a single-reactor system gave only a yield of 69.2% chlorohydrin, with more undesired products.
In the double-chamber reaction system, chlorine is dissolved in a recycle stream of dilute aqueous propylene chlorohydrin together with makeup water. From this vessel, the effluent is passed to a second chamber, into which the propene is fed.
Modern plants boast propylene chlorohydrin yields of 87-90%, with ongoing efforts to push efficiency beyond 90%. Several patented technologies focus on optimizing reaction conditions and minimizing dichloride formation.
A novel method that employs tertiary alkyl hypochlorites (e.g., tert-butyl hypochlorite) for the hypochlorination of propene is described in the following reactions:
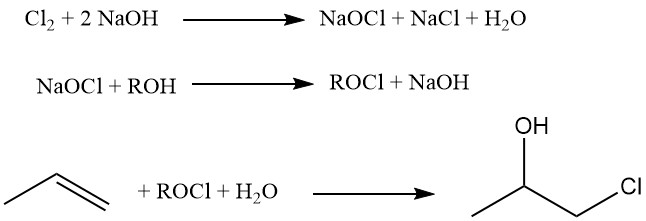
This method benefits from the low solubility of this hypochlorite in water, enabling near-chloride-free environments that drastically reduce dichloride formation. This process has achieved propylene chlorohydrin selectivities exceeding 95%.
4. Uses of Propylene Chlorohydrin
Propylene chlorohydrin is used in the production of propylene oxide, an essential building block for various industrial applications (polyurethane polyols and propylene glycol).
5. Toxicology of Propylene Chlorohydrin
Propylene chlorohydrins, particularly 1-chloro-2-propanol and 2-chloro-1-propanol, pose moderate toxicity through various exposure routes. Understanding their health hazards is essential for safe handling and use.
General Risks:
- Moderate Toxicity: Exposure via ingestion, inhalation, or skin contact can lead to adverse effects.
- Skin and Eye Irritation: Both compounds significantly irritate the skin and eyes upon contact.
Specific Exposure Effects:
- Oral: LD50 values (oral dose lethal to 50% of animals) for rats range from 190 to 270 mg/kg, indicating moderate toxicity.
- Dermal: The LD50 for rabbits is about 480 mg/kg, again suggesting moderate toxicity. Skin irritation is mild.
- Inhalation: The LC50 (inhalation concentration lethal to 50% of animals) for 1-chloro-2-propanol is 1000 ppm for 4 hours, signifying acute toxicity at high concentrations. Subacute exposure can cause lung congestion and edema.
- Repeat-Dose: Chronic exposure at high doses can affect various organs, including the liver, kidneys, pancreas, and reproductive system, in both rats and mice.
- Teratogenicity: Limited evidence suggests weak teratogenic potential in rats.
- Mutagenicity: Both pure and technical-grade propylene chlorohydrin exhibit mutagenic activity in several tests.
- Carcinogenicity: Studies in rats and mice exposed to technical-grade mixtures did not show carcinogenic effects.
- Human Epidemiology: One study found no increased cancer risk in workers exposed to propylene chlorohydrin production processes.
Classification and Exposure Limits:
- GHS classification: flammable liquid, toxic by ingestion, inhalation, and skin contact, skin irritant, and causes serious eye damage.
- ACGIH TLV-TWA: 1 ppm (8-hour exposure limit).
References
- Chlorohydrins; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_565.pub2
- https://link.springer.com/chapter/10.1007/978-1-4613-8719-0_2