Trichloroacetic acid (TCA), also known as trichloroethanoic acid, is a colorless hygroscopic crystalline solid with the chemical formula Cl3CCOOH. It is a strong acid and a corrosive substance that can damage skin, eyes, and other tissues upon contact.
Table of Contents
1. Physical Properties of Trichloroacetic Acid
Trichloroacetic acid [76-03-9] exists as hygroscopic, rhombohedral crystals at standard temperature and pressure with a pungent odor. It has high solubility in water and various organic solvents. Key physical properties include:
- Molar mass: 163.4 g/mol
- Melting point: 59.2 °C
- Boiling point: 197.6 °C at 101.3 kPa; ~107 °C under reduced pressure (2.8 kPa)
- Density at 60 °C: 1.63 g/cm³
- Refractive index at 65 °C: 1.459
- pKa: 0.70
- Dipole moment: 3.23 D
2. Chemical Properties of Trichloroacetic Acid
Trichloroacetic acid exhibits strong acidity (pKa = 0.7) due to the complete substitution of methyl hydrogens with chlorine atoms, making it more stable than chloroacetic and dichloroacetic acids and less susceptible to substitution reactions of chlorine atoms.
Under aqueous conditions, elevated temperatures induce the decomposition of trichloroacetic acid into chloroform and carbon dioxide. This process is accelerated in the presence of organic or inorganic bases, while aniline, resorcinol, and activated carbon serve as catalysts in anhydrous conditions.
Purely thermal decomposition occurs only above the boiling point and yields chlorinated hydrocarbons, carbon monoxide, carbon dioxide, and phosgene.
Trichloroacetic acid forms salts with diverse inorganic and organic bases. These salts decompose upon heating in aqueous environments, releasing chloroform.
3. Production of Trichloroacetic Acid
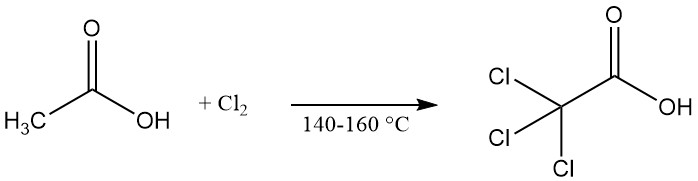
Trichloroacetic acid is commercially produced by the chlorination of acetic acid, chloroacetic acid, or its mother liquors at elevated temperatures (140–160 °C). Calcium hypochlorite can be used as a catalyst to accelerate the reaction.
The use of heavy metal salts as catalysts remains controversial. While examples like iron and copper compounds have been utilized, concerns regarding their removal by precipitation with sulfuric or phosphoric acid persist.
Alternative approaches explored include using 2% phosphoric acid or combining catalysts with UV light. Catalyst-free methods have also been developed.
The resulting crude product, typically containing around 95% trichloroacetic acid, undergoes purification through melt crystallization. This effectively removes impurities present in the mother liquor. Further enhancement of purity can be achieved via centrifugation or additional recrystallization steps.
When sulfuric acid is used as a catalyst at higher reaction temperatures (up to 180 °C), post-production purification is not necessary.
4. Uses of Trichloroacetic Acid
Prior to the 1990s, the primary application of trichloroacetic acid was the production of its sodium salt, used as a selective herbicide and in combination with 2,4-D and 2,4,5-T formulations for total weed control. However, due to ecotoxicological concerns, this application has diminished significantly.
Beyond herbicides, trichloroacetic acid finds diverse uses in various fields:
- In metal surface treatment, trichloroacetic acid is employed as etching and pickling agent
- It is used as a swelling agent and solvent in the plastics industry.
- In Biochemistry, For the precipitation of proteins, albumin, DNA, and RNA
- Auxiliary agent in textile finishing
- It is added as an additive to enhance the high-pressure properties of lubricating oils.
- Due to its corrosive nature, trichloroacetic acid is employed in controlled settings to remove warts, hard skin and treat specific skin conditions.
- Trichloroacetic acid and its esters serve as valuable starting materials for various organic syntheses.
- The acid exhibits distinctive color reactions, enabling the identification of diverse organic compounds in analytical chemistry.
5. Derivatives of Trichloroacetic Acid
5.1. Trichloroacetyl chloride
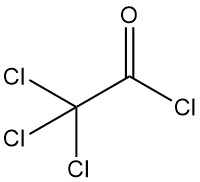
Trichloroacetyl chloride [76-02-8] has the chemical formula Cl3CCOCl and a molar mass of 197.9 g/mol, possesses physical properties similar to dichloroacetyl chloride:
- Boiling point: 118°C at 101.3 kPa
- Density at 20°C: 1.620 g/cm³
- Refractive index at 20°C: 1.4695
Trichloroacetyl chloride readily hydrolyzes to trichloroacetic acid and hydrochloric acid at 75–85 °C in water. It reacts with ammonium hydroxide or concentrated sodium carbonate solutions to form corresponding salts.
Trichloroacetyl chloride is produced by various methods:
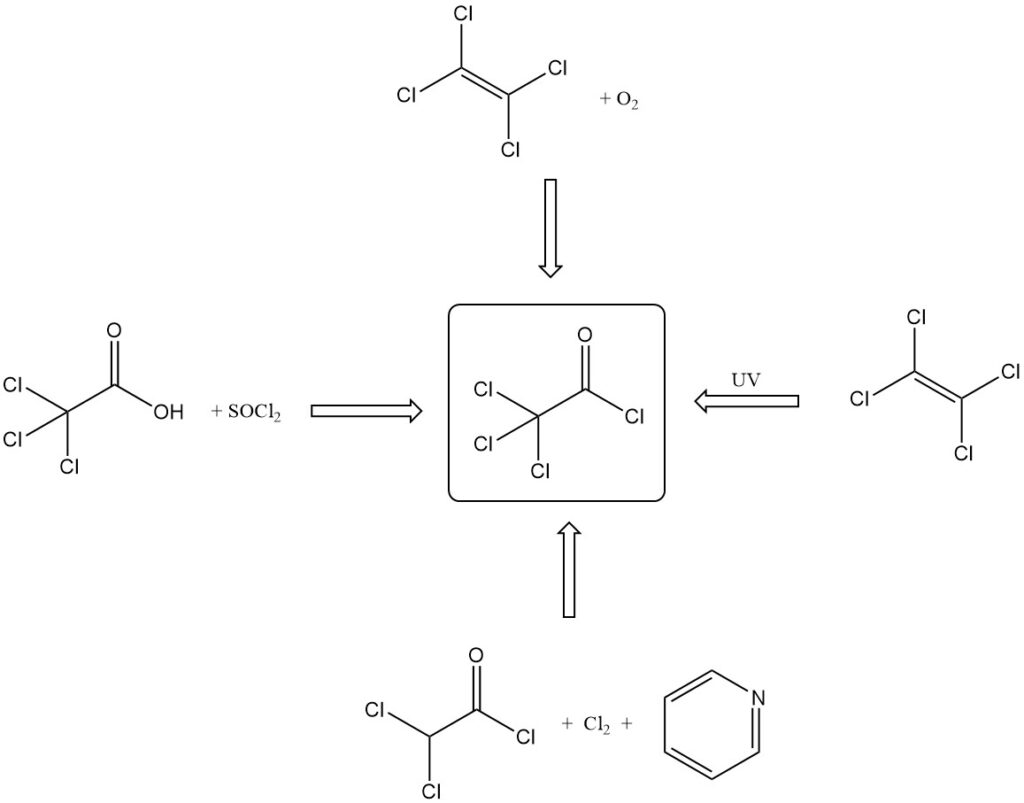
- By reaction of trichloroacetic acid with inorganic acid chlorides (SOCl2, PCl3) or P2O5 and HCl.
- By tetrachloroethylene oxidation with fuming sulfuric acid, oxygen, or fuming nitric acid and sulfuric acid.
- By photochemical oxidation of tetrachloroethylene with UV light, radioactive irradiation, or sensitization with chlorine/iodine.
- By the reaction of dichloroacetyl chloride with pyridine and chlorine.
Trichloroacetyl chloride is used to produce trichloroacetic acid esters, anhydrides, and pesticides (e.g., Chlorpyrifos) and as a starting material for the synthesis of trifluoroacetic acid.
5.2. Trichloroacetic Acid Esters
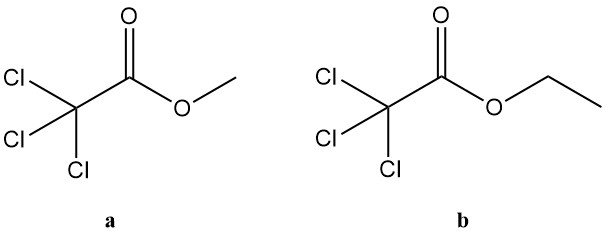
Trichloroacetic acid esters encompass a broad group of compounds, but only the methyl and ethyl derivatives hold significant industrial interest. These two esters find application in various areas:
- Solvents: While not their primary use, both methyl and ethyl trichloroacetate can function as niche solvents when necessary.
- These esters serve as valuable starting materials for the synthesis of other compounds, particularly amides and polyalcohol esters. These derivatives have been explored for their potential use as plasticizers, offering specific functionalities or properties not readily available in conventional plasticizers.
- Ethyl trichloroacetate finds wider application as a co-catalyst in Ziegler-type polymerizations.
For reference, here are some key physical properties of the two industrially relevant esters:
a. Methyl trichloroacetate:
- CAS number: 598-99-2
- Formula: Cl3CCOOCH3
- Molar mass: 177.43 g/mol
- Boiling point: 153 °C (at 101.3 kPa)
- Density: 1.4864 g/cm³ (at 20 °C)
- Refractive index: 1.4572 (at 20 °C)
b. Ethyl trichloroacetate:
- CAS number: 515-84-4
- Formula: Cl3CCOOCH2CH3
- Molar mass: 191.45 g/mol
- Boiling point: 167.5 °C (at 101.3 kPa)
- Density: 1.3823 g/cm³ (at 20 °C)
- Refractive index: 1.4505 (at 20 °C)
5.3. Sodium Trichloroacetate
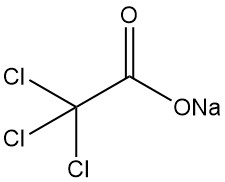
Sodium trichloroacetate (Cl3CCOONa), the only industrially significant salt of trichloroacetic acid, demonstrates several key properties:
Physical properties:
- A colorless solid decomposes below its melting point.
- Highly soluble in water and methanol, moderately soluble in other polar solvents.
- Solubility in water increases with temperature (50% at 5 °C, 60% at 20 °C, 70% at 40 °C).
Chemical properties:
- Stable when dry but undergoes hydrolysis in an aqueous solution, generating sodium bicarbonate and dichloroacetic acid.
- Thermal decomposition at elevated temperatures (125–170 °C) produces sodium chloride, trichloroacetyl chloride, carbon monoxide, and carbon dioxide.
- It reacts with olefins if phase-transfer catalysts are used.
Production:
Sodium trichloroacetate is industrially produced by neutralizing trichloroacetic acid with sodium hydroxide or carbonate.
Applications:
- Sodium trichloroacetate, combined with reducing agents and metal salts, promotes vinyl polymerization.
- It aids dye absorption and dispersal in polyester and cellulose fibers.
- In diazo paper developed by heat, It is used to liberate base at elevated temperatures (100–200 °C)
- It was previously used as an herbicide to control monocotyledonous weeds, with a soil half-life of 1-2 months; however, concerns over ecotoxicity led to its widespread bans in the USA, Canada, EU, and South America.
6. Toxicology of Trichloroacetic Acid and Trichloroacetate
Trichloroacetic acid poses several health risks due to its corrosive nature and protein precipitation ability. While not readily absorbed through the skin, it can cause significant eye and skin irritation upon contact.
Key Toxicity Data:
- Oral LD50: 3320–5060 mg/kg (rats)
- Lowest lethal dose (female dogs): 1590 mg/kg
- Inhalation (4 hours, high dose): No significant effects observed in animals
- Acute human exposure: Skin and eye burns, irritation, gastrointestinal effects
- Chronic data: Limited, potentially affects upper respiratory tract
- Proposed TLV-TWA: 1 ppm (7 mg/m³)
Concerns for the safety of trichloroacetic acid include:
- Skin and eye damage: Its corrosive properties can cause burns and irritation.
- Protein binding: displaces drugs from protein-binding sites, potentially altering their effectiveness.
- Limited chronic data: Potential long-term effects require further investigation.
Trichloroacetate has a provisionally tolerated daily intake (PTDI) of 0.075 mg/kg body mass. This value is based on a subchronic dog feeding study, where various adverse effects were observed at doses of ≥ 2000 mg/kg in food.
Adverse effects were observed, such as loss of body mass, malaise, tissue damage (gums, mouth lining), changes in white blood cells, abnormal urine findings, liver and heart lesions, muscle atrophy, and impaired sperm production.
The nontoxic effect level was 500 mg/kg in the diet. In vitro data suggests no mutagenic potential.
Reference
- Chloroacetic Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_537.pub3