Isopropyl Alcohol: Properties, Chemical Reactions, Industrial Production, Applications, and Toxicology
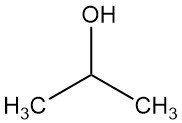
What is isopropyl alcohol?
Isopropyl alcohol, also known as isopropanol or 2-propanol, is the simplest secondary alcohol with the chemical formula C3H7OH. It is a colorless, volatile, and flammable liquid that has a a slightly bitter taste and a faint odor similar to a mixture of ethanol and acetone.
Isopropanol is found in nature in crude fusel oils and as fermentation and decomposition products of various vegetables. It is regarded as the first petrochemically derived alcohol and among lower alcohols (C1–C5), it ranks third in global production after methanol and ethanol.
Table of Contents
1. Physical properties of isopropyl alcohol
Isopropyl alcohol (2-propanol) is a secondary alcohol that is completely miscible with water and readily soluble in a wide range of common organic solvents, including ethers, esters, ketones, acids, and other alcohols.
Isopropanol solubility in water decreases in the presence of dissolved salts, and the addition of salts such as sodium chloride or sodium sulfate induces phase separation, producing an immiscible organic layer. This property is exploited in the extraction of polar compounds from aqueous solutions.
The physical properties of isopropyl alcohol are influenced by its polar hydroxyl (–OH) group and its secondary alcohol structure. It has boiling and flash points lower than n-propanol, while its vapor pressure and freezing point are higher.
Table 1 provides the physical properties of anhydrous isopropyl alcohol as well as its 91 vol% azeotropic mixture with water.
| Property | Anhydrous | 91 Vol % |
|---|---|---|
| Molecular weight | 60.10 | |
| boiling point, at 101.3 kPa, °C | 82.3 | 80.4 |
| freezing point, °C | −88.5 | −50.0 |
| specific gravity, 20/20 | 0.7864 | 0.8183 |
| density, at 20°C, g/cm3 | 0.7854 | 0.8173 |
| surface tension, at 20°C, mN/m(= dyn/cm) | 21.32 | 21.40 |
| specific heat, liquid at 20 °C, J/(kg·K) | 2510.4 | |
| refractive index | 1.3772 | 1.3769 |
| heat of combustion, at 25 °C, kJ/mol | 2005.8 | |
| latent heat of vaporization, at 101.3 kPa, kJ/mol | 39.8 | |
| vapor pressure, at 20 °C, kPa | 4.4 | 4.5 |
| critical temperature, °C | 235.2 | |
| critical pressure, at 20 °C, kPa | 4764 | |
| viscosity, mPa·s(= cP) at 0 °C | 4.6 | |
| viscosity, mPa·s(= cP) at 20 °C | 2.4 | 2.1 |
| viscosity, mPa·s(= cP) at 40 °C | 1.4 | |
| flammability limit in air, vol % | lower: 2.5 upper: 12 |
|
| flash point, °C (open cup) | 17.2 | 21.7 |
| flash point, °C(closed cup) | 11.7 | 18.3 |
| autoignition temperature, °C | 399 |
Commercial grades of isopropanol in the United States are predominantly anhydrous isopropyl alcohol and a 91 vol% aqueous azeotrope known as the constant boiling mixture. Rubbing alcohol formulations typically contain 70 % or more isopropyl alcohol in water.
Isopropyl alcohol also forms azeotropes with various compounds, including hydrocarbons, esters, halocarbons, amines, ketones, and aromatic hydrocarbons. It does not form binary azeotropes with acetone, ethanol, ethylbenzene, hexylamine, or methyl isobutyl ketone, but it can form ternary azeotropic systems with some of these compounds.
Examples of some isopropyl alcohol binary azeotropes are given in Table 2.
| Component | Boiling point, °C | Azeotrope boiling point, °C | Isopropyl alcohol composition, wt % |
|---|---|---|---|
| water | 100 | 80.3 | 87.4 |
| toluene | 110.6 | 80.6 | 69 |
| methyl propionate | 79.6 | 77 | 28 |
| methyl ethyl ketone | 79.6 | 77.9 | 32 |
| ethyl acetate | 77.05 | 75.9 | 25 |
| 2-chlorobutane | 68.25 | 64 | 18 |
| hexane | 68.9 | 62.7 | 23 |
| cyclohexane | 80.8 | 68.6 | 33 |
| butylamine | 77.8 | 84.7 | 60 |
| diisopropyl ether | 69 | 66.2 | 16.3 |
2. Chemical reactions of isopropyl alcohol
The chemical properties of isopropyl alcohol are determined by the presence of a secondary hydroxyl group. Its reactivity is characteristic of secondary alcohols and is generally higher at the hydroxyl moiety compared with primary alcohols such as n-propyl or ethyl alcohol. Most reactions involve cleavage of either the C–OH or the O–H bond.
Isopropyl alcohol undergoes a wide range of transformations, including dehydrogenation, oxidation, esterification, etherification, amination, and halogenation. Industrially important examples include the preparation of aluminum isopropoxide and isopropyl halides.
Aluminum isopropoxide is produced in quantitative yield by refluxing isopropyl alcohol with aluminum metal in the presence of catalytic amounts of mercuric chloride:
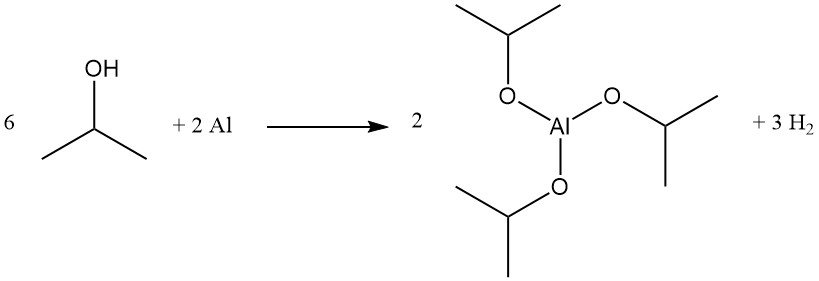
This compound is an effective Meerwein–Ponndorf–Verley reducing agent for certain ester exchange and carbonyl reduction reactions and is used as a precursor for aluminum glycinate, a buffering agent.
Isopropyl halides, such as isopropyl bromide, are obtained by displacement of the hydroxyl group through reaction with hydrogen halides. Refluxing isopropyl alcohol with hydrobromic acid yields isopropyl bromide and water:
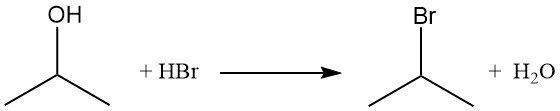
The order of reactivity with hydrogen halides is HI > HBr > HCl. The reaction with hydrochloric acid to form isopropyl chloride is catalyzed by the presence of zinc chloride.
Isopropyl alcohol can be deprotonated to yield isopropoxide salts, which are widely used as strong, non-nucleophilic bases in organic synthesis.
In catalytic transfer hydrogenation reactions, it is used as a terminal reductant for the reduction of aldehydes, ketones, and other functional groups. During these reactions, 2-propanol is oxidized to acetone.
This process is reversible, and extended contact between the product and catalyst can decrease enantiopurity in asymmetric reductions. The equilibrium can be shifted toward product formation by using an excess of isopropanol, by removing acetone through distillation, or by separating the reduced product from the reaction medium.
2.1. Dehydrogenation of isopropyl alcohol
Dehydrogenation of isopropyl alcohol is an important industrial reaction for producing acetone. Prior to the large-scale co-production of acetone in phenol manufacturing processes, it was the primary commercial method for acetone synthesis.
The process is endothermic, with a heat requirement of 66.5 kJ/mol at 327 °C. It is carried out in the vapor phase using temperatures of 300–500 °C and moderate pressures around 207 kPa to give ca. 90% selectivity to acetone and 98% 2-propanol conversion.
Catalysts commonly used include copper, chromium, zinc, and nickel, applied either individually, as oxides, or in mixed forms supported on inert materials. A representative reaction is:

Selectivity toward acetone is high, but small quantities of by-products are formed through dehydration, condensation, or oxidation pathways. These include propylene, diisopropyl ether, mesityl oxide, acetaldehyde, and propionaldehyde.
The reaction can also proceed in the liquid phase.
Dehydrogenation of isopropyl alcohol can also be performed oxidatively using silver or copper catalysts at temperatures of 400–600 °C. Patented processes describe vapor-phase oxidative dehydrogenation for the production of mixed ketones, including acetone, methyl isobutyl ketone, and higher ketones.
In one example, vapor-phase dehydrogenation of an azeotropic mixture of 2-propanol and water over a copper-based catalyst at 220 °C produces a mixture containing acetone (52.4%), unreacted 2-propanol (11.4%), methyl isobutyl ketone (21.6%), diisobutyl ketone (6.5%), and 4-methyl-2-pentanol (2.2%).
2.2. Oxidation of isopropyl alcohol
Isopropyl alcohol undergoes catalytic oxidation with air or oxygen at 400–600 °C to produce acetone and water:
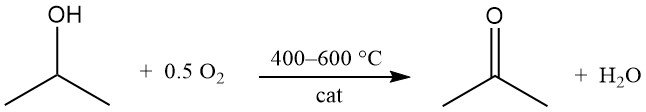
Catalysts are similar to those used in dehydrogenation processes. Unlike dehydrogenation, the oxidation reaction is highly exothermic and releases 180 kJ/mol at 295 °C. Careful control of process conditions is necessary to limit by-product formation, particularly from dehydration pathways.
Oxidation and dehydrogenation can be carried out simultaneously with appropriate catalyst and operating conditions, although industrial use of oxidation for acetone production is limited compared with dehydrogenation.
Partial oxidation of isopropyl alcohol can be performed in the liquid phase without a catalyst to produce hydrogen peroxide and acetone:
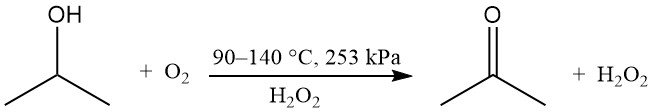
Oxygen or air is used with a peroxide initiator, such as hydrogen peroxide. The reaction rate is influenced by the concentration of by-product acetic acid. The theoretical weight ratio of acetone to hydrogen peroxide is 1.7. This method is used when hydrogen peroxide is the desired product, while acetone and unreacted isopropanol are recycled.
In Shell’s process, hydrogen peroxide produced this way is used to oxidize allyl alcohol to acrolein, while in the Burmah Oil process it is converted to peracetic acid.
2-propanol also reacts with α,β-unsaturated aldehydes or ketones over metal oxide catalysts at elevated temperatures. In a Shell process for allyl alcohol production, a vapor-phase mixture of isopropanol and acrolein is passed over uncalcined magnesium oxide and zinc oxide at 400 °C to yield approximately 77 % allyl alcohol based on acrolein.
2.3. Esterification of isopropyl alcohol
Isopropyl alcohol undergoes esterification with carboxylic acids in the presence of acidic catalysts such as p-toluenesulfonic acid. The reaction is typically performed at 100–160 °C and atmospheric pressure, using an excess of alcohol to drive the equilibrium toward ester formation.
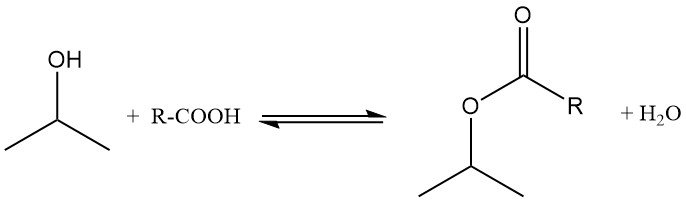
Water formed during the reaction is removed as an azeotrope to shift the equilibrium. Excess isopropanol is distilled and recycled, and ester yields approach quantitative levels. For example, isopropyl acetate is prepared from isopropanol and acetic acid in the presence of sulfuric acid, with toluene used as the azeotroping agent.
Kinetic studies have been conducted on esterification of isopropanol with various organic acids using sulfonated cation-exchange resins. Reaction with myristic acid yields isopropyl myristate, an emollient and lubricant used in cosmetics and topical pharmaceuticals. A jellied form of this ester is marketed as Estergel.
Isopropyl alcohol reacts with carbon disulfide to produce xanthate esters. Sodium isopropyl xanthate is used in mineral flotation processes and as a herbicide in bean and pea cultivation.
The reaction of 2-propanol with phosphorus halides forms phosphite esters. Triisopropyl phosphite is obtained from phosphorus trichloride and isopropyl alcohol at low temperatures in the presence of an acid scavenger such as pyridine:
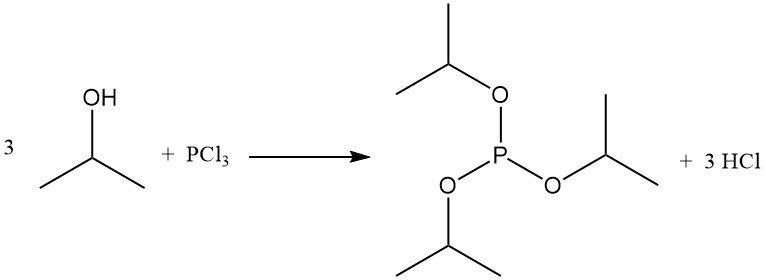
Titanium tetrachloride reacts with isopropanol to produce tetra(isopropyl) titanate, a commercial polymerization catalyst.
Isopropyl nitrate is prepared by the reaction of isopropanol with nitric acid, with the reactants fed separately into a still and the product continuously removed by distillation.
Isopropyl nitrate is used as an engine-starting fuel and in explosive formulations. The nitrite ester, isopropyl nitrite, is formed from isopropanol and either nitrosyl chloride or nitrous acid at ambient temperature and serves as a jet engine propellant.
2.4. Etherification of isopropyl alcohol
Isopropyl alcohol can undergo dehydration in the liquid phase over strong acidic catalysts, such as sulfuric acid, or in the vapor phase over acidic alumina to produce either diisopropyl ether (DIPE) or propylene.
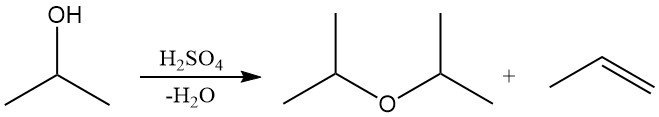
The selectivity toward DIPE or propylene depends on the choice of catalyst and reaction conditions. Industrially, DIPE is mainly obtained as a by-product during isopropyl alcohol production. Excess DIPE is often recycled over acidic catalysts to regenerate isopropyl alcohol via hydration. DIPE is used in limited quantities as a solvent and in industrial extraction processes.
The 1990 U.S. Clean Air Act, which mandated the use of oxygenates in gasoline, created potential demand for DIPE as a fuel oxygenate. DIPE can be synthesized by reacting isopropyl alcohol with propylene over acidic ion-exchange catalysts at low temperatures.
Glycol ethers can also be prepared from isopropyl alcohol by reaction with olefin oxides such as ethylene oxide or propylene oxide. For example, reaction with ethylene oxide yields 2-isopropoxyethanol (commercially known as isopropyl Cellosolve), typically catalyzed by alkali hydroxides.
Secondary alkoxylation of the initial product with additional olefin oxide forms higher alkoxylated derivatives (oligomers). This competing reaction is particularly significant when ethylene oxide is used, as the terminal primary hydroxy group produced is more reactive.
Glycol ethers derived from isopropyl alcohol are used as solvents in lacquers, enamels, and waterborne coatings to enhance gloss, flow, and film properties.
2.5. Amination of isopropyl alcohol
Isopropyl alcohol undergoes amination by ammonolysis with dehydration catalysts or by reductive ammonolysis using hydrogenation catalysts. Both methods produce isopropylamine and diisopropylamine. Triisopropylamine is formed only in negligible amounts.
The ratio of mono- to diisopropylamine depends on the molar ratio of isopropyl alcohol to ammonia, which typically ranges from 2:1 to 5:1.
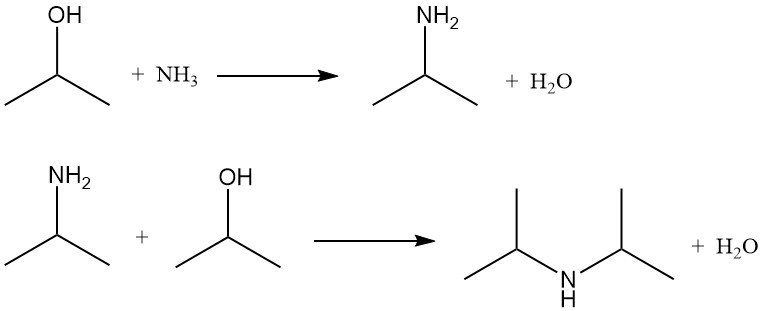
In reductive ammonolysis, hydrogen is present to prolong catalyst life by preventing coking and tar formation but is not significantly consumed. The reactions is normally conducted at 150–250 °C and 790–2860 kPa in a fixed-bed reactor using catalysts consisting of copper, chromium, or nickel supported on alumina.
The conversion of isopropyl alcohol exceeds 85%, and amine yields are greater than 90%. By-products such as nitriles and amides are recycled to improve yield.
Direct ammonolysis with dehydration catalysts is carried out at 300–500 °C and similar pressures. Catalysts used include alumina, silica, titanium dioxide, and aluminum phosphate. Yields exceed 80%, with minimal coking and nitrile formation, but control over amine composition is limited.
Isopropylamine is mainly used in herbicide production, particularly for 2-chloro-4-ethyl-6-isopropylamino-s-triazine, and to a lesser extent in pesticide manufacturing. Diisopropylamine is used in pesticides and as a corrosion inhibitor, such as diisopropylammonium nitrate.
2.6. Halogenation of isopropyl alcohol
2-Halopropane derivatives can be synthesized from isopropyl alcohol by reaction with the corresponding acid halide, which is the most economical method. Phosphorus halides and elemental halogens can also react under suitable conditions to replace the hydroxyl group and produce the halide.
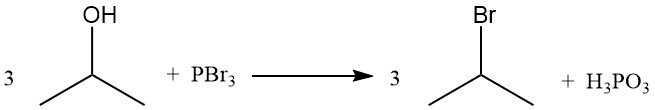
Halogenation in aqueous solution is accompanied by oxidation. Chlorination at 65 °C produces chloroacetone derivatives, mainly 1,3-dichloroacetone and 1,1,3-trichloroacetone. Further chlorination at 70–100 °C leads to almost complete conversion to higher chlorinated products, including 1,1,1,3,3-pentachloroacetone and hexachloroacetone.
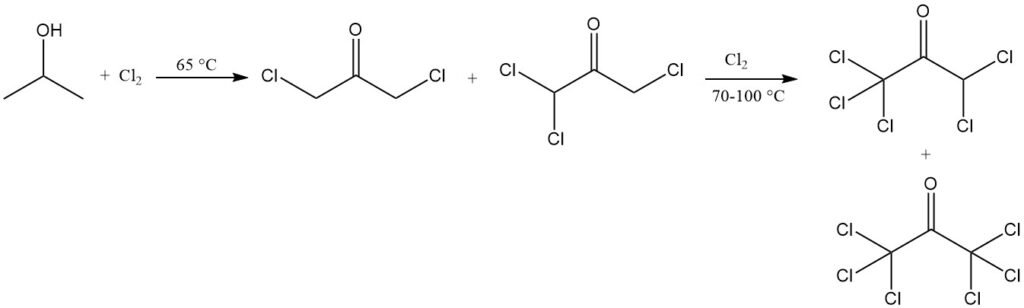
Chlorination of isopropanol under specific conditions can yield 1,1,1,3-tetrachloroacetone, which can be converted to 1,1,1-trichloro-2,3-epoxypropane, an intermediate for agricultural and pharmaceutical chemicals, as well as low-flammability plastics.
2.7. Miscellaneous reactions of isopropanol
Isopropyl alcohol undergoes acylation with ketene to produce isopropyl acetate:
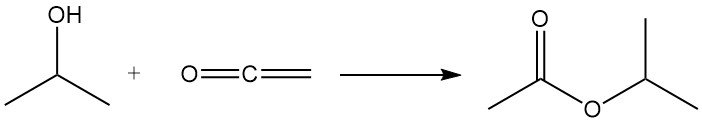
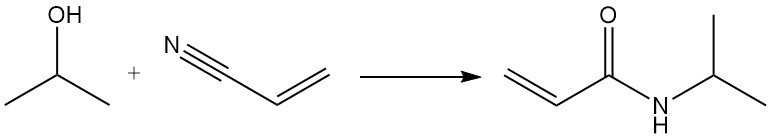
The Ritter reaction of isopropanol with acrylonitrile yields N-isopropylacrylamide:
Isopropyl alcohol can also condense with aromatic compounds such as toluene and phenol to form isopropoxylated derivatives that are used as intermediates in the synthesis of resins, surfactants, and specialty chemicals.
3. Industrial production of isopropyl alcohol
Isopropyl alcohol is produced industrially by two major processes: indirect hydration and direct hydration of propene. Smaller volumes are obtained by hydrogenation of acetone.
Industrial-scale manufacture of isopropyl alcohol began in 1920 at the Bayway, New Jersey petrochemical plant of Standard Oil (Exxon). In 1921, Union Carbide (Carbide and Carbon Chemicals Corporation) initiated production in Clendenin, West Virginia. Shell Oil Company started operations in the 1930s at Dominguez, California. These companies remained the principal U.S. producers into the mid-1990s.
The indirect hydration process, also known as the sulfuric acid process, was historically the only commercial method until 1951, when Imperial Chemical Industries (ICI) commissioned the first commercial direct hydration process.
Both methods use propylene and water as feedstocks. The indirect process can accommodate refinery C3 off-gas streams containing 40–60 wt % propylene, making it suitable for U.S. refinery integration. The direct hydration route was developed to overcome issues of corrosion, high energy consumption, and air emissions associated with the indirect method; however, it requires high-purity propylene.
Other potential synthetic methods include fermentation of carbohydrates, oxidation of propane, and hydrolysis of isopropyl acetate. Of these, hydrogenation of by-product acetone from phenol production is the only alternative process of commercial significance.
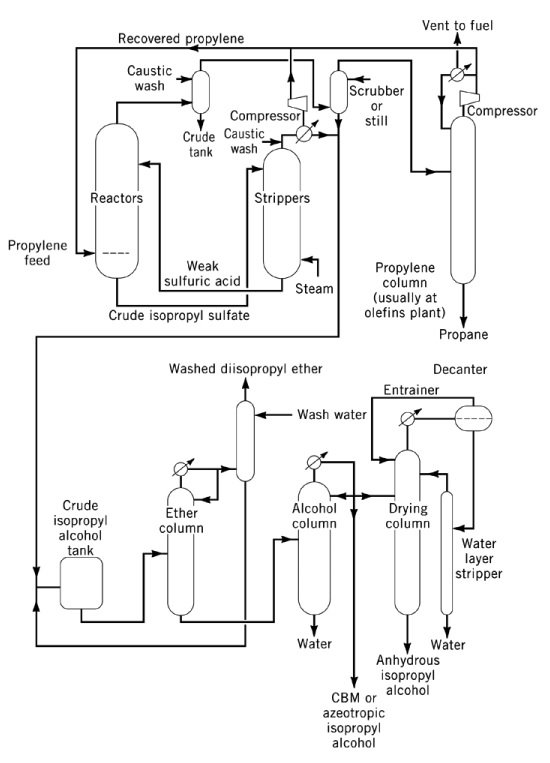
3.1. Production of isopropyl alcohol by indirect hydration of propylene
Indirect hydration of propylene to isopropyl alcohol is a two-step process that uses concentrated sulfuric acid as the catalyst. In the first step, esterification occurs to form sulfate esters, primarily isopropyl hydrogen sulfate, along with smaller amounts of diisopropyl sulfate. In the second step, hydrolysis of these esters yields isopropanol and regenerates sulfuric acid.
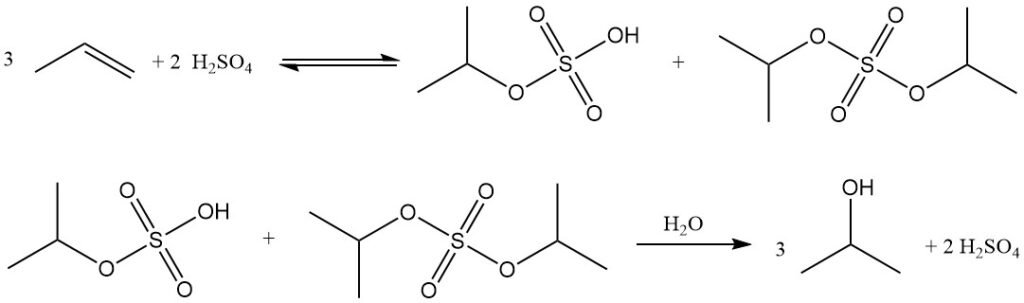
The major by-product of this process is diisopropyl ether (DIPE), formed by the reaction of intermediate sulfate esters with isopropanol. Other by-products include acetone (from thermal decomposition of sulfate esters), mesityl oxide, propylene oligomers (dimers, trimers), and minor alcohols such as ethanol, 2-butanol, and n-propanol from feed impurities.
Sulfur-containing compounds in combination with unsaturated oligomers can cause undesirable odors, which are mitigated by optimized reaction conditions and post-treatment over metals such as copper or nickel.
The reaction is conducted at 0.7–2.8 MPa and 20–80 °C using sulfuric acid concentrations above 60 wt %. Two operational modes are common:
- The strong acid process is a two-step operation that uses >80 wt % H2SO4 at 1–1.2 MPa and 20–30 °C.
- The weak acid process is single-stage operation that uses 60–80 wt % H2SO4 at approx. 2.5 MPa and 60–65 °C.
Both processes achieve more than 98 % selectivity to 2-propanol and DIPE.
The hydrolysate is stripped to yield a mixture of isopropanol, DIPE, and water overhead, with dilute sulfuric acid as bottoms. DIPE is generally recycled to the reactor for hydration. Wet isopropanol (87 wt %, 91 vol %) is obtained after distillation, with more than 93 % conversion of propylene. Final dehydration to anhydrous grade is carried out by azeotropic distillation using DIPE or cyclohexane as entrainers.
Corrosion control requires steel reactors for concentrated acid at moderate temperatures, and stainless steel, tantalum, or Hastelloy for dilute acid at higher temperatures. High-purity or essence-grade isopropyl alcohol is produced by post-treatment over activated carbon, molecular sieves, or metal-containing fixed beds, followed by final distillation in nonferrous equipment.
Indirect hydration remains the primary commercial route in the United States and is also practiced by several European and Japanese producers.
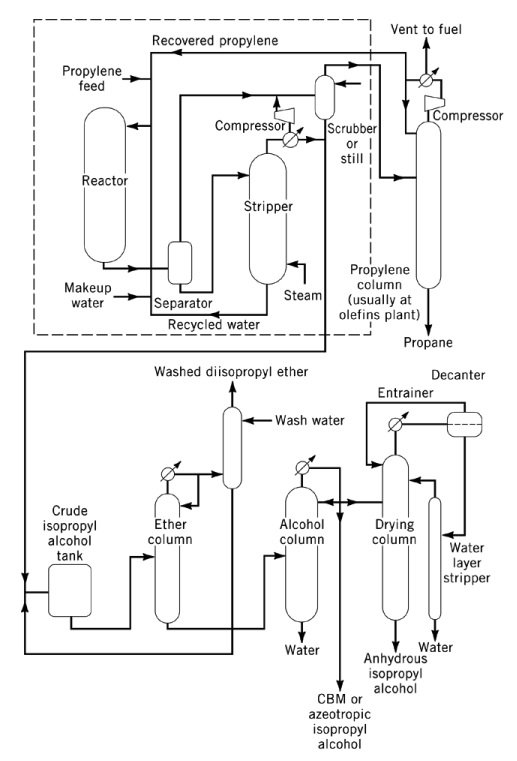
3.2. Production of isopropyl alcohol by direct hydration of propylene
The direct hydration of propylene to 2-propanol is an acid-catalyzed, exothermic reaction with a heat of reaction of −50 kJ/mol. Thermodynamically, high pressures and low temperatures favor the formation of the isopropyl alcohol, although most catalysts require moderate to high temperatures to achieve commercial yields.
Commercial direct hydration processes are classified into three principal types: vapor-phase hydration, mixed vapor–liquid-phase hydration, and liquid-phase hydration.
In vapor-phase processes, such as the Veba-Chemie and ICI processes, propylene and water vapor are passed over a fixed-bed acidic catalyst. The Veba-Chemie process uses phosphoric acid supported on silica at 240–260 °C and 2.5–6.6 MPa to achieve 96 % selectivity with 5–6 % conversion per pass.
The ICI process uses a tungsten oxide–zinc oxide catalyst on silica at 230–290 °C and 20.3–25.3 MPa. In both cases, the high temperature and low pressure limit conversion therefore necessitate extensive propylene recycling. Feedstock purity requirements are typically around 99 wt %.
Mixed vapor–liquid-phase hydration, developed by Deutsche Texaco AG, is conducted in a trickle-bed reactor using a sulfonic acid cation-exchange resin catalyst. The reaction operates at 130–160 °C and 8–10 MPa with a propylene-to-water molar ratio of 1:12–15.
This process achieves over 75 % conversion per pass and 93 % selectivity when the feedstock purity is at least 92 wt %. By-products formed during the reaction include approximately 5 % diisopropyl ether (DIPE) and minor amounts of higher oligomeric alcohols. Catalyst lifetime exceeds eight months under normal operation.
Liquid-phase hydration, as developed by Tokuyama Soda, uses a weakly acidic aqueous silicotungstate catalyst at 270 °C and 20.3 MPa. The process yields 60–70 % conversion per pass with 98–99 mol % selectivity.
The catalyst is recycled with minimal loss, and the process exhibits low corrosion and negligible emissions. Feedstock purity is 95 wt % or higher. The crude isopropanol product, obtained by azeotropic distillation, contains 88 wt % isopropanol and is subsequently purified to over 99.99 % purity.
Catalysts used in direct hydration are strong acids capable of protonating propylene to form a secondary carbocation. Examples include supported phosphoric acid, tungsten oxide, molybdophosphoric acid, titanium oxide, zinc oxide, zirconium oxide, silicotungstates, molybdenum oxalate, and various zeolites.
Strong acidic ion-exchange resins are preferred for moderate-temperature operation. Certain polyacid anions, such as phosphomolybdate and phosphotungstate, have been shown to enhance hydration rates compared to sulfate or phosphate, possibly due to specific ion association effects with the carbocation.
The reaction mechanism involves protonation of propylene to generate a secondary carbonium ion, followed by nucleophilic attack by water to yield protonated isopropyl alcohol, which subsequently deprotonates to form the neutral alcohol. The rate of reaction is proportional to the hydronium ion concentration and independent of the counter-anion, although polyacid anions can accelerate the rate.
From a thermodynamic perspective, the equilibrium constant in the vapor phase is given by log K = 2624/T- 7.584, and in the liquid phase, the standard free energy is ΔF° = 23.25T- 9352 (where T is in K).
Low temperatures reduce the formation of DIPE, but catalyst activity sets a practical lower limit. High pressures increase the alcohol concentration and reduce the need for extensive propylene recycling.
3.3. Production of isopropyl alcohol by hydrogenation of acetone
Hydrogenation of acetone is employed in the United States, Russia, Asia, and South America. The process can be carried out in the liquid phase over a fixed-bed Raney nickel catalyst and achieves 99.9 % selectivity and 99.9 % conversion of acetone.
Alternatively, hydrogenation over a copper oxide–chromium oxide catalyst at 120 °C and 196 kPa gives lower selectivities of about 98 % and conversions of approximately 94 %. High-purity acetone is not required for this process, making it particularly advantageous in facilities where excess acetone is generated as a by-product, such as in the cumene-phenol process.
4. Applications of isopropyl alcohol
Isopropyl alcohol (2-propanol) is used primarily as an industrial solvent, chemical intermediate, and antiseptic.
4.1. Uses of isopropyl alcohol in the chemical industry
Major chemical derivatives of isopropanol include methyl isobutyl ketone (MIBK), methyl isobutyl carbinol (MIBC), diisobutyl ketone (DIBK), and isopropyl acetate. 2-propanol is also a feedstock for the production of isopropylamines, which are used in the manufacture of herbicides and pesticides. Some diisopropylamine is used in the synthesis of diisopropylammonium nitrate, a corrosion inhibitor.
The use of isopropyl alcohol for acetone production has declined significantly due to the prevalence of the cumene oxidation process.
4.2. Uses of isopropyl alcohol as a solvent
As a solvent, isopropyl alcohol has balanced alcohol, water, and hydrocarbon-like properties, making it effective for dissolving oils, gums, waxes, resins, and alkaloids. It is used in the formulation of cements, primers, varnishes, paints, printing inks, coatings, and extraction processes.
In cosmetics and personal care products, it is used as a solvent for lotions, perfumes, shampoos, skin cleansers, nail polishes, makeup removers, deodorants, and body oils, with fragrance added to mask its odor.
Aerosol formulations containing isopropyl alcohol include hair sprays, cleaning products, insecticides, automotive deicers, air fresheners, disinfectants, and various household and industrial sprays.
4.3. Medical usage of isopropyl alcohol
In the pharmaceutical sector, isopropyl alcohol is used as a processing solvent and as an active antiseptic and disinfectant in home, hospital, and industrial settings. It is more effective than ethanol for microbial control and is widely used in 70% aqueous solutions as rubbing alcohol.
It is also present in medicinal liniments, tinctures, scalp tonics, surgical suture baths, and dressing solutions, as well as in formulations such as tincture of iodine and green soap.
4.4. Other uses
High-purity isopropyl alcohol (99.99%) is used in the electronics industry for cleaning large-scale integration (LSI) devices and silicon wafers. Additional applications include its use as an octane enhancer, carburetor anti-icing additive, and cosolvent with methanol in gasoline blends.
In extraction processes, aqueous solutions of isopropyl alcohol are applied to liquid–liquid extraction of fatty acids from vegetable oils at low temperatures.
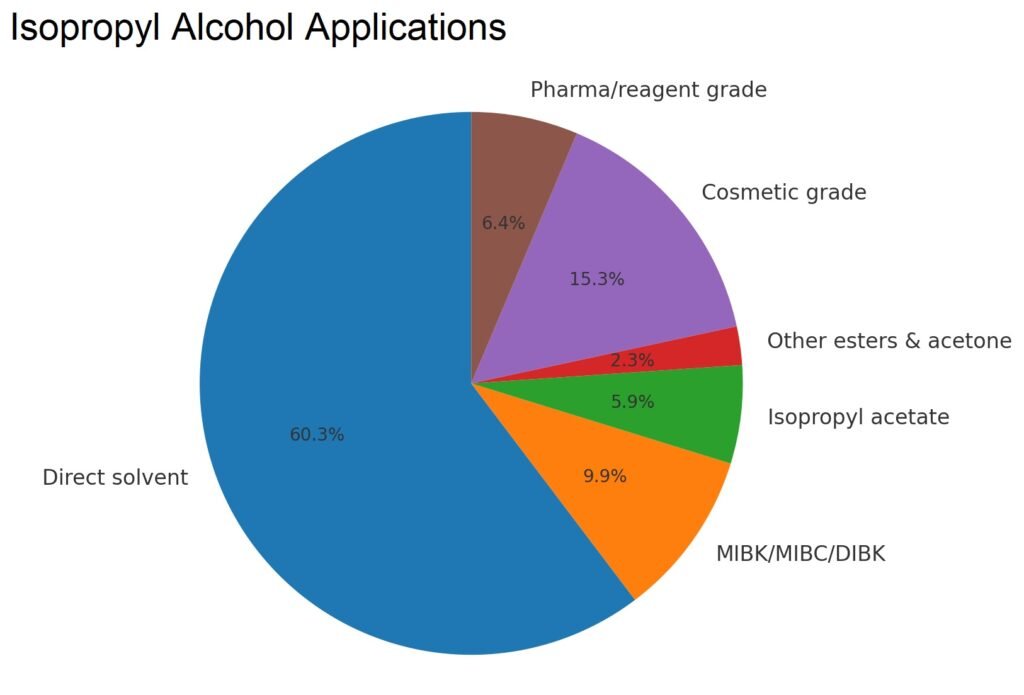
U.S. consumption data in Table 3 show primary uses of isopropanol in solvents, coatings, chemical derivatives, cosmetics, pharmaceuticals, and specialty applications, with trends indicating a decline in acetone and isopropylamine production but stable use in cosmetics, pharmaceuticals, and high-performance solvents.
| Year | |||
|---|---|---|---|
| 2002 | 2008 | 2014 | |
| Direct solvent | 243 | 269 | 237 |
| Chemical derivatives | |||
| MIBK/MIBC/DIBK | 71 | 45 | 39 |
| Isopropylamines | 85 | 50 | – |
| Isopropyl acetate | 24 | 17 | 23 |
| Other esters and acetone | 17 | 17 | 9 |
| Cosmetic grade | 52 | 56 | 60 |
| Pharma (USP grade)/reagent grade | 21 | 25 | 25 |
| Total | 513 | 479 | 393 |
5. Toxicology of isopropyl alcohol
Isopropyl alcohol is widely present in household and industrial products, including rubbing alcohol, disinfectants, cleaners, cosmetics, pharmaceuticals, and solvents. Its toxicity is higher than ethanol, approximately twice as great, but lower than methanol. The primary exposure route is ingestion, with less frequent cases from inhalation or dermal exposure.
Isopropyl alcohol is rapidly absorbed after ingestion, with peak plasma concentrations occurring within 30 minutes. It is widely distributed in the body water, with a volume of distribution of 0.45–0.55 L/kg.
Metabolism occurs primarily in the liver by alcohol dehydrogenase to acetone, acetol, methylglyoxal, propylene glycol, acetate, and formate, with subsequent conversion into glucose and other metabolic products.
Acetone is the major metabolite and appears in the breath within 15 minutes after ingestion. Elimination occurs mainly via the kidneys, with 20% excreted unchanged and the remainder as acetone and its metabolites. The elimination half-life of isopropyl alcohol is 2.5–8 hours, while acetone elimination is slower, with a half-life of 7.7–27 hours.
The mechanism of toxicity is predominantly brainstem depression caused by both isopropyl alcohol and, to a lesser extent, acetone. Clinical features range from mild intoxication to severe central nervous system (CNS) and respiratory depression, circulatory collapse, shock, and coma. Early symptoms include ataxia, vomiting, abdominal pain, and hematemesis, progressing in severe cases to hypotension, hypothermia, and loss of reflexes.
Unlike methanol and ethylene glycol, isopropyl alcohol and acetone do not cause high anion gap metabolic acidosis but produce ketonemia, ketonuria, and an elevated osmolar gap. A fruity odor on the breath is characteristic.
The toxic dose of 70% isopropyl alcohol is approximately 1 mL/kg, and ingestion of 100–250 mL may be fatal. Death results from CNS paralysis. Inhalation of high vapor concentrations can cause anesthetic effects and mild irritation of the eyes, nose, and throat.
Occupational exposure limits are set by OSHA and ACGIH at 400 ppm (8-h TWA) and 500 ppm (STEL). The odor threshold ranges from 3 to 200 ppm. Industrial use under normal conditions does not pose a significant health risk.
Animal studies, including subchronic inhalation, neurotoxicity, developmental, reproductive, and oncogenicity testing, have not demonstrated adverse effects.
Diagnosis of isopropanol poisoning is based on exposure history, clinical findings, and laboratory results showing osmolar gap with ketosis in the absence of metabolic acidosis. Measurement of serum isopropyl alcohol levels can confirm exposure but is not always available.
Management of isopropanol poisoning is primarily supportive to maintain vital functions, including airway protection, cardiovascular stabilization (to address potential hypotension), and monitoring of the patient’s acid–base and electrolyte balance. Activated charcoal is ineffective in this type of poisoning and therefore not recommended.
Hemodialysis enhances elimination of both isopropyl alcohol and acetone but is generally reserved for severe, life-threatening cases with profound hypotension, coma, or very high serum concentrations. Most patients recover fully with prompt supportive care.
References
- Klabunde, J., Bischoff, C. and Papa, A.J. (2025). Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a22_173.pub3
- Logsdon, J.E. and Loke, R.A. (2000). Isopropyl Alcohol. In Kirk-Othmer Encyclopedia of Chemical Technology, (Ed.). https://doi.org/10.1002/0471238961.0919151612150719.a01
- Jolliff, H.A. (2017). Isopropyl Alcohol. In: Brent, J., et al. Critical Care Toxicology. Springer, Cham. https://doi.org/10.1007/978-3-319-17900-1_53
- Co, I. N.; Gunnerson, K. J. Chapter 71 – Iatrogenic and Poison-Derived Acid Base Disorders. In Critical Care Nephrology, 3rd ed.; Ronco, C., Bellomo, R., Kellum, J. A., Ricci, Z., Eds.; Elsevier, 2019; pp 417-423.e2. DOI: 10.1016/B978-0-323-44942-7.00071-6
- Gwaltney-Brant, S. M. Chapter 24 – Miscellaneous Indoor Toxicants. In Small Animal Toxicology, 3rd ed.; Peterson, M. E., Talcott, P. A., Eds.; W.B. Saunders, 2013; pp 291-308. DOI: 10.1016/B978-1-4557-0717-1.00024-7
- Slaughter, R. J., Mason, R. W., Beasley, D. M. G., Vale, J. A., & Schep, L. J. (2014). Isopropanol poisoning. Clinical Toxicology, 52(5), 470–478. https://doi.org/10.3109/15563650.2014.914527
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100F.) ISOPROPYL ALCOHOL MANUFACTURE BY THE STRONG-ACID PROCESS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK304434/
- Carls, R.-R.; Osterburg, G.; Prezelj, M.; Webers, W. “Process for the production of isopropyl alcohol.” U.S. Patent 4,760,203, 1988. Available from: https://patents.google.com/patent/US4760203A/en
- Hirata, S.; Ogawa, S. “Process for producing isopropyl alcohol.” U.S. Patent 5,763,693, 1998. Available from: https://patents.google.com/patent/US5763693A/en
- Gershbein, L. L.; Pines, H.; Ipatieff, V. N. “Reactions of Isopropyl Alcohol in the Presence of Catalysts Containing Magnesium Oxide.” J. Am. Chem. Soc., 1947, 69 (11), 2888–2893. DOI: 10.1021/ja01203a074
