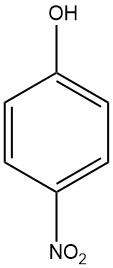
4-Nitrophenol, also known as p-nitrophenol or 4-hydroxynitrobenzene, is an aromatic compound with the chemical formula C6H5NO3. It is a yellow to colorless crystalline solid with a sweet, phenolic odor. It is soluble in water, ethanol, and other organic solvents.
Table of Contents
1. Physical Properties of 4-Nitrophenol
P-nitrophenol has a metastable α-form that can be observed in crystalline form when toluene is heated above 63 °C. The more stable β-form is obtained when toluene is cooled below 63°C. P-nitrophenol is not readily volatile with steam, but it is more soluble in water than its ortho-isomer.
Some of the physical properties of 4-nitrophenol are presented in the following table:
| Property | Value |
|---|---|
| Chemical formula | C6H5NO3 |
| Molar mass | 139.11 g/mol |
| Appearance | Yellow, needle-like crystalline solid |
| Melting point | 114 °C |
| Boiling point | 216 °C |
| Solubility in water (100 °C) | 30% |
| Solubility in water (25 °C) | 16 g/L |
| Density | 1.48 g/cm³ |
| pKa | 7.15 |
| Flash point | 105 °C |
| Sodium salt decomposition temperature | 156 °C |
2. Chemical Reactions of 4-Nitrophenol
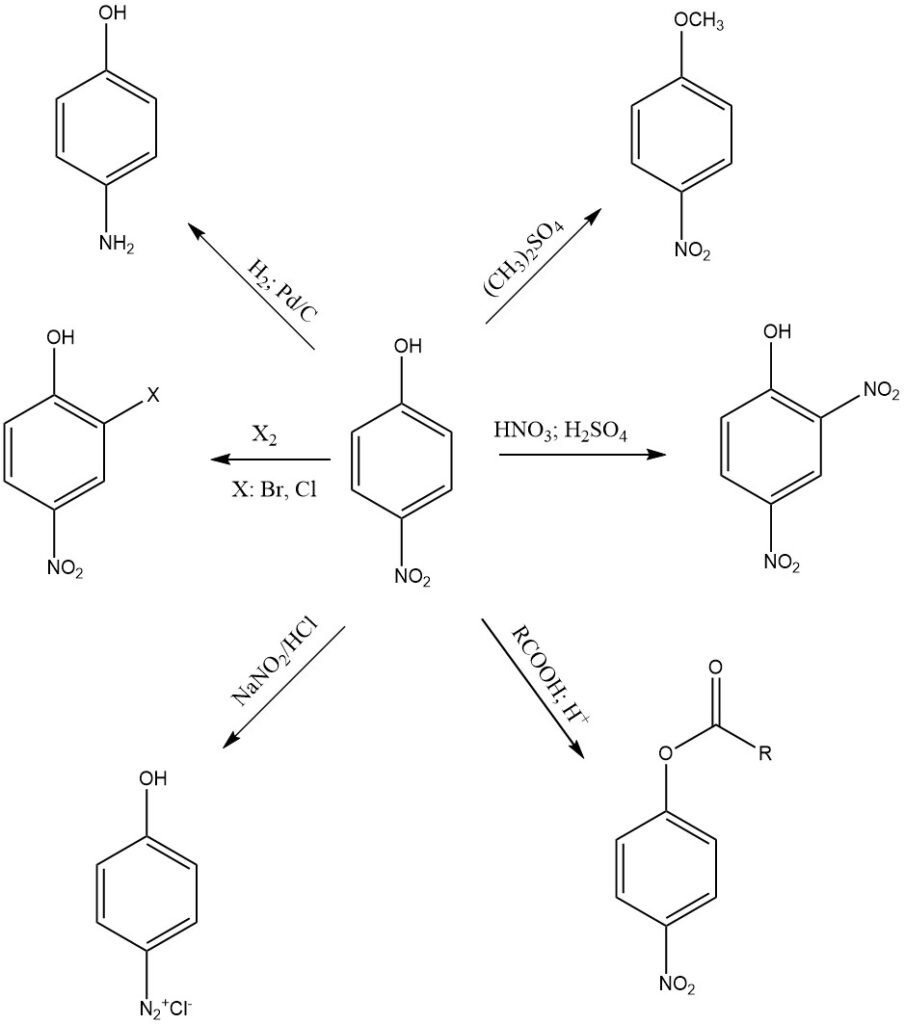
4-Nitrophenol is a relatively stable compound. Its reactivity is contributed to the presence of nitro and hydroxyl groups on the aromatic ring, so it can undergo a variety of chemical reactions, as follows:
Reduction
One of the most common reactions of 4-nitrophenol is reduction of the nitro group to amine. 4-nitrophenol can be reduced to 4-aminophenol under a variety of conditions. Common reducing agents include sodium dithionite, zinc, and iron in concentrated HCl or hydrogen gas.
Alkylation
4-nitrophenol can be alkylated to form a variety of derivatives. Common alkylating agents include dimethyl sulfate and methyl iodide. Alkylated 4-nitrophenol derivatives are often used as intermediates in the synthesis of other organic compounds.
Esterification
4-Nitrophenol can undergo esterification, where it reacts with carboxylic acids to form esters. This reaction is typically catalyzed by acid, such as sulfuric acid.
Nitration
4-nitrophenol is nitrated in the presence of nitric acid and sulfuric acid to form 2,4-dinitrophenol. Further nitration yields picric acid, which is a more powerful explosive than 2,4-dinitrophenol, and it is used in a variety of industrial and military applications.
Diazotization
4-Nitrophenol can also participate in diazotization reactions, a process that converts the amino group (-NH2) of 4-aminophenol into a diazonium salt (-N2+X–). This reaction is used in the synthesis of various dyes and aromatic compounds with diazo groups.
Acid-Base Reactions
4-nitrophenol is acidic because of its hydroxyl group. It reacts with strong bases like sodium hydroxide to form 4-nitrophenolate ions.
4-nitrophenol also undergoes aromatic ring reactions such as halogenation, sulfonation, acylation, and alkylation, particularly in the ortho position of the (-OH) group.
3. Production of 4-Nitrophenol
3.1. Hydrolysis of 4-chloronitrobenzene
4-chloronitrobenzene is heated in an autoclave with 8.5% sodium hydroxide solution. The exothermic reaction reaches 170 °C and is maintained for 8 hours. The solution is then cooled and acidified to yield 4-nitrophenol in 95% yield.

The process produce high purity 4-nitrophenol and includes the following steps:
- Basic hydrolysis of the 4-chloronitrobenzene
- Concentration of the reaction medium
- Acidification to obtain the 4-nitrophenol compound from its salt
- Liquid/liquid decantation to remove the aqueous phase obtained after acidification
- Crystallization of the 4-nitrophenol compound
- Separation of the product
The concentration step removes volatile impurities and the decantation step removes water-soluble impurities. The crystallization step further purifies the 4-nitrophenol by removing any remaining impurities.
This process produces a 4-nitrophenol with a low content of liposoluble impurities (such as 4-chloronitrobenzene) and water-soluble impurities (such as sodium chloride and sodium sulfate). 4-Nitrophenol is also free from sulfurous impurities, which is advantageous for subsequent catalytic hydrogenation reactions.
3.2. Nitration of phenol
Phenol is reacted with nitric acid in the presence of sulfuric acid to produce a mixture of 2-nitrophenol and 4-nitrophenol. The mixture is then distilled to separate the two isomers. The 4-nitrophenol obtained from the distillation step is further purified by crystallization or recrystallization.

A more efficient process for producing 4-nitrophenol from phenol in a nitric acid medium is a methode involves two steps: nitrosation and nitration. In the nitrosation step, phenol is reacted with nitrous acid to produce p-nitrosophenol.
In the nitration step, p-nitrosophenol is reacted with nitric acid to produce 4-nitrophenol. The process is carried out in a nitric acid medium of concentration in the range of 5 to 30% by weight, and the temperature is maintained in the range of 0-10 ° C during the nitrosation and 15-30 ° C during the nitration.
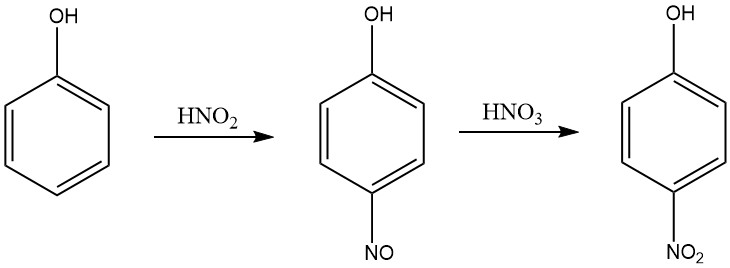
The nitrous acid is produced in the aqueous nitric acid by addition of oxides of nitrogen, such as nitric oxide, nitrogen tetroxide, or nitrogen trioxide. The 4-nitrophenol is separated from the reaction mixture by crystallization.
This process is advantageous because it produces 4-nitrophenol with a low ortho isomer content.
4. Uses of 4-nitrophenol
The reduction of 4-nitrophenol to 4-aminophenol is less used because processes to directly reduce nitrobenzene are more common.
4-Nitrophenol reacts with dialkylthiophosphoric chlorides at 125 °C in chlorobenzene to form a group of insecticides including parathion. But this compound has been replaced by less toxic alternatives like methylparathion and related substances.
4-Nitrophenol reacts with 4-chloro-3-nitrobenzotrifluoride to form fluorodifen, which is related to nitrofen. This herbicide is used to treat drilled rice crops.
4-Nitrophenol is used in other applications including:
- Pharmaceuticals: 4-nitrophenol is used as an intermediate in the synthesis of paracetamol (also known as acetaminophen), a common over-the-counter pain reliever and fever reducer.
- Fungicides: 4-nitrophenol is a precursor for some fungicides, such as dinocap and binapacryl.
- Dyes: 4-nitrophenol is used to produce a variety of dyes, including yellow, orange, and red dyes.
- Leather tanning: 4-nitrophenol is used to darken leather.
5. Toxicology of 4-nitrophenol
| Exposure | LD50 |
|---|---|
| Dermal, guinea pig | >1 gm/kg |
| Oral, mouse | 282 mg/kg |
| Oral, rat | 202 mg/kg |
| Skin, rat | 1024 mg/kg |
| Inhalation, rat | > 4.7 mg/l/4H |
| Skin sensitization, guinea pig | None sensitized |
- Acute effects: Inhalation or ingestion of 4-nitrophenol can cause headaches, drowsiness, nausea, and cyanosis (blue color in lips, ears, and fingernails). Contact with the eyes can cause irritation.
- Chronic effects: No information is available on the chronic effects of 4-nitrophenol in humans or animals from inhalation or oral exposure.
- Reproductive/developmental effects: No information is available on the reproductive or developmental effects of 4-nitrophenol in humans.
- Cancer risk: No information is available on the carcinogenic effects of 4-nitrophenol in humans. There was no evidence of carcinogenic activity in mice dermally exposed to 4-nitrophenol for 18 months in a National Toxicology Program (NTP) study.
References
- Nitro Compounds, Aromatic, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a17_411
- Process for preparation of a nitrophenol. – https://patents.google.com/patent/US20080045756A1/en
- Preparation of p-nitrophenols. – https://patents.google.com/patent/US3510527A/en
- https://www.epa.gov/sites/default/files/2016-09/documents/4-nitrophenol.pdf
- https://fscimage.fishersci.com/msds/96371.htm


