Methanol, also known as methyl alcohol, is a simple alcohol with the chemical formula CH3OH. It is a colorless, flammable liquid with a slightly sweet odor. Methanol is the simplest alcohol, and it is the most widely produced alcohol in the world.
Methanol is used as a fuel, a solvent, and a building block for many other chemicals and is also a renewable energy source.
Table of Contents
1. Use of Methanol for Chemical Syntheses
About 70% of global methanol production is used as a raw material in various chemical syntheses. The most important applications of methanol are formaldehyde, methyl tert-butyl ether (MTBE), acetic acid, dimethyl ether (DME), propene, methyl methacrylate, and dimethyl terephthalate (DMT).
Methanol is also increasingly being used for energy and fuel purposes, either directly or as a precursor material, especially in emerging economies.
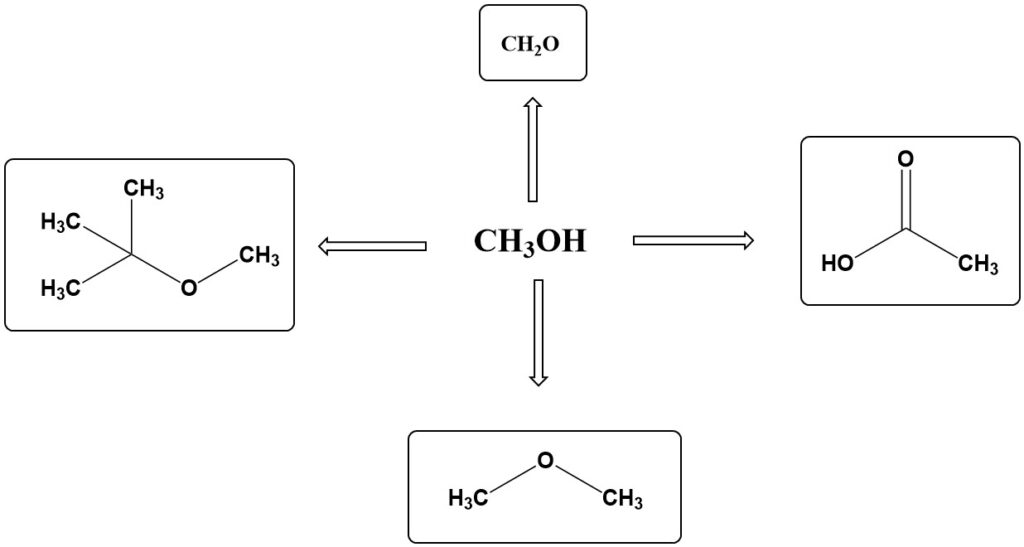
Formaldehyde from Methanol
Formaldehyde is the most important product made from methanol. In 2011, about 28% of global methanol production was used to make formaldehyde. Although formaldehyde production has continued to grow in recent years, its share of methanol consumption has decreased due to faster growth in other sectors, such as propene and fuel applications.
Formaldehyde is made from methanol by oxidizing it with atmospheric oxygen. The specific process used depends on the temperature and type of catalyst employed.
Methyl tert-Butyl Ether (MTBE) is produced by the reaction of methanol with isobutene using acid ion exchangers. This is an excellent octane enhancer and gained prominence with the introduction of unleaded gasoline grades and increased awareness of the potential harm associated with aromatic high-octane components in recent decades.
Because of safety concerns, such as MTBE release from high vapor pressure storage tanks, this product has faced resistance, particularly in Western countries in recent years. As a result, methanol consumption for MTBE production has decreased from 27% in 1996 to a mere 11% in 2011 and there is a shift in commercial interest towards ethyl tert-butyl ether (ETBE) as a substitute for MTBE.
The overall production of MTBE is anticipated to rise again due to increased usage and production capacities in emerging economies, such as Asia and the Middle East.
Acetic Acid accounts for 11% of methanol use, with estimated annual growth rates of 4% until 2013. Acetic acid is synthesized by the carbonylation of methanol with carbon monoxide in the liquid phase using homogeneous catalysts, including cobalt–iodine, rhodium–iodine, or nickel–iodine.
The older BASF process operates at 65 MPa, while more modern processes, such as the Monsanto process, operate at 5 MPa. By adjusting operating conditions, the synthesis can also be modified to produce acetic anhydride or methyl acetate.
Methanol is employed in various processes to produce gasoline, olefins, propene, and aromatic compounds as alternative fuels. These developments emerged as a response to the oil crisis and involve the conversion of synthesis gas into fuels using methanol as an intermediate.
One notable process is methanol-to-gasoline (MTG) synthesis, with the New Zealand government and Mobil operating a plant that produces 4,500 t/d of methanol from natural gas, subsequently converting it into 1,700 t/d of gasoline.
Other significant synthesis routes include methanol-to-olefins (MTO), methanol-to-propene (MTP), and methanol-to-aromatic compounds (MTA) processes. The first two technologies have been successfully employed in recent years.
The first DMTO (DICP methanol-to-olefins, Shenhua Baotou) and MTP (Lurgi) plants were commissioned in 2010/2011, consuming 3 million tons of methanol in 2011. This alternative synthesis route in China allows the production of propene–polypropylene based on coal as the sole carbon source.
Dimethyl Ether (DME) has emerged as an alternative to chlorofluorocarbons. It serves as a better propellant for sprays than propane–butane mixtures with its higher polarity and superior solubilizing power for spray products.
DME is used as a solvent, organic intermediate, and in adhesives. It accounts for approximately 7% of methanol consumption. When integrated with conventional large-scale methanol plants, the DME production process benefits from operational efficiency, resulting in DME production capacities exceeding 3,000 tons per day.
Methanol is the precursor for various other organic compounds, including formic acid, methyl esters of organic acids, methyl esters of inorganic acids, methylamine, trimethylphosphine, sodium methoxide, methyl halides, and ethylene, each serving specific industrial purposes, such as solvents, monomers, catalysts, and organic intermediates.
2. Uses of Methanol as Energy Source

Methanol is a promising alternative to petroleum-derived fuels, especially as coal’s significance in emerging economies grows. Coal-to-methanol synthesis and Fischer–Tropsch are the most promising chemical utilization routes for coal.
Methanol, along with downstream products like MTBE, DME, or MTG-gasoline, can provide valuable energy and fuel sources. Some envision a ‘methanol economy’ based on methanol derived from diverse sources, including coal, residues, biogas, CO2, or other carbon sources, capable of satisfying the full spectrum of requirements for future transportation and energy applications.
2.1. Methanol as a Fuel for Otto Engines
Methanol has been considered as a motor fuel since the 1920s, mainly in high-performance racing cars and airplanes. Research into methanol combustion in four-stroke engines is ongoing. Methanol has several advantages as a fuel.
Its high heat of vaporization and relatively low calorific value result in significantly lower combustion chamber temperatures compared to conventional motor fuels. This contributes to reduced emissions of nitrogen oxides, hydrocarbons, and carbon monoxide, although it is accompanied by increased formaldehyde emissions.
- M3 is a blend of 3% methanol with 2–3% solubilizers in commercially available motor fuel. No modifications to motor vehicles and fuel distribution systems are required.
- M15 is a mixture of 15% methanol and a solubilizer with motor fuel, necessitating vehicle alterations. The initial proposal to use M15 to enhance the octane number in unleaded gasoline was largely replaced by the widespread adoption of MTBE.
- M85 is methanol containing 15% C4–C5 hydrocarbons to improve cold-start properties. Modified vehicles and fuel distribution systems are essential.
- M100 is pure methanol. Vehicles must undergo substantial modifications and be fully adapted to methanol operation.
2.2. Methanol as Diesel Fuel
Exclusive operation with methanol in diesel engines is unfeasible due to the low cetane number of methanol, which results in unreliable ignition. Consequently, methanol must be converted into downstream products like DME, which serves as an excellent diesel alternative. When combined with suitable fats and oils, methanol yields fatty acid methyl esters (FAMEs), commonly known as biodiesel.
2.3. Other Energy Uses of Methanol
Methanol has been discussed and implemented in pilot projects for firing peak-load gas turbines in power stations, also known as “peak shaving.” This approach is advantageous due to the simple storage of methanol and its environmentally friendly combustion in gas turbines.
Methanol, as well as DME, has demonstrated potential as a future fuel for stationary turbine engines. Furthermore, using methanol as a fuel in conventionally fired boilers has the potential to obviate the need for costly flue gas treatment facilities, although its economic viability remains a challenge.
Gasification of methanol to produce synthesis gas or fuel gas has been proposed but has often been hindered by economic concerns.
However, the chemical conversion of CO2 into methanol using hydrogen generated via water electrolysis is gaining recognition as a promising method for producing renewable fuels or utilizing methanol as a liquid energy carrier. Though the concept is not novel and has been previously discussed, it is now receiving greater attention and undergoing active investigation.
3. Other Uses of Methanol
Methanol’s unique properties, such as its low freezing point and ability to mix with water, make it useful in a variety of applications:
Refrigerants: Methanol is used in refrigeration systems, both pure (e.g., in ethylene plants) and mixed with water and glycols. It is also used as an antifreeze in heating and cooling circuits. Compared to other common antifreezes like ethylene glycol, propylene glycol, and glycerol, methanol has lower viscosity at low temperatures. However, it is no longer used as an engine antifreeze, replaced by glycol-based products.
Natural Gas Pipeline Protection: Large quantities of methanol are used to protect natural gas pipelines from gas hydrate formation at low temperatures. Methanol is injected into natural gas at pumping stations, transported as a liquid in the pipeline, and then recovered at the pipeline’s end. It can be recycled after removing water absorbed from natural gas by distillation.
Gas Scrubbers: Methanol is used as an absorption agent in gas scrubbers to remove CO2 and H2S at low temperatures (e.g., Rectisol process by Linde and Lurgi). An advantage of this process is that even traces of methanol remaining in the purified gas typically do not interfere with subsequent processing.
Solvent Applications: While pure methanol is not widely used as a solvent, it is often included in solvent mixtures to improve their properties and expand their applications.
Reference
- Methanol; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_465.pub3


