4-Aminophenol Derivatives
Table of Contents
4-(N-Methylamino)phenol

4-(N-Methylamino)phenol, with the chemical formula C7H9NO and a molecular weight of 123.15 g/mol, exhibits needle-like crystalline structures when precipitated from benzene.
Its melting point is 87 °C, and it has a boiling range of 168 to 169 °C at a pressure of 2 kPa. This substance has slight solubility in alcohol and remains insoluble in ether.
The industrial synthesis of 4-(N-Methylamino)phenol involves the decarboxylation process of N-(4-hydroxyphenyl)glycine at elevated temperatures, employing solvents like chlorobenzene-cyclohexanone.
An alternative synthetic route is the methylation of 4-aminophenol or the reaction of methylamine with 4-chlorophenol and copper sulfate in an aqueous solution at 135 °C, or with hydroquinone in an alcoholic solution at 200 to 250 °C.
Primarily, 4-(N-Methylamino)phenol serves as a crucial component in photographic developers. Due to its susceptibility to degradation in the presence of air and light, it is typically marketed as the sulfate salt, known as Metol, which has a decomposition temperature of 260 °C.
Additionally, it finds application as an intermediate compound in the production of fur and hair dyes and may act as a corrosion inhibitor for steel under specific conditions.
Prolonged exposure to 4-(N-methylamino)phenol has been linked to the development of dermatitis and allergic reactions.
4-(N,N-Dimethylamino)phenol
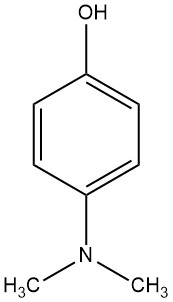
4-(N,N-Dimethylamino)phenol, is characterized by its large rhombic crystals, which can be obtained from either ether-hexane or ether-ligroin crystalization mixtures. They have a melting point of 75 to 76 °C and a boiling point range of 101 to 103 °C at 66.7 Pa.
The compound readily forms a salt with sulfuric acid which displays a melting range of 208 to 210 °C.
The synthesis of 4-(N,N-Dimethylamino)phenol involves the methylation of 4-aminophenol using a methyl halide under pressure.
An alternative approach involves dealkylation of 4-methoxydimethylaniline by reflux with hydroiodic acid for 10 hours.
Another production method is the photodecomposition of 4-dimethylaminobenzenediazonium tetrafluoroborate.
4-(N,N-Dimethylamino)phenol serves as an intermediate in various synthetic reactions and has recently gained prominence in experimental toxicity studies involving animals. It has been shown to induce methemoglobinemia.
4-Hydroxyacetanilide
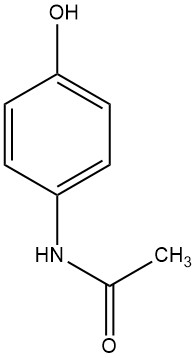
4-Hydroxyacetanilide, identified by its chemical names 4-acetamidophenol, acetaminophen, or paracetamol, forms a large white monoclinic prisms when precipitated from water, displaying a melting range of 169 to 171 °C. It is an odorless compound with a bitter taste.
It is insoluble in petroleum ether, pentane, and benzene; slight soluble in ether and cold water; and solubility in hot water, alcohols, dimethylformamide, 1,2-dichloroethane, acetone, and ethyl acetate.
The compound’s pKa value is 9.5 at 25 °C.
The production of 4-Hydroxyacetanilide involves the acetylation of 4-aminophenol. This can be achieved by various methods, such as using acetic acid and acetic anhydride at 80 °C, acetic anhydride in pyridine at 100 °C, acetyl chloride and pyridine in toluene at 60 °C, or the reaction with ketene in an alcoholic suspension.
An alternative synthesis route is the direct transformation from 4-nitrophenol. Reduction-acetylation approaches include tin with acetic acid, hydrogenation over Pd-C in acetic anhydride, and hydrogenation over platinum in acetic acid.
Other pathways include the rearrangement of 4-hydroxyacetophenone hydrazone with sodium nitrite in sulfuric acid and the electrolytic hydroxylation of acetanilide.
Beyond its role as an intermediate in the production of azo dyes and photographic chemicals, 4-Hydroxyacetanilide has antipyretic and analgesic properties, finding extensive use in medical applications. Its oral LD50 in rats is recorded as 3.7 g/kg.
4-Methoxyaniline

4-Methoxyaniline has a molecular formula of C7H9NO and a molecular weight of 123.55 g/mol, a melting point of 58.5 °C and a boiling point range of 123.2 to 123.5 °C at a pressure of 1.9 kPa.
It possesses a dipole moment of 1.8 Debye (benzene). Its refractive index is 1.5559 at 67 °Cand has a density of 1.092 at 55 °C. The compound exhibits a viscosity of 3.215 millipascal seconds (mPa·s) at 55 °C.
For its industrial production, 4-Methoxyaniline is synthesized by the reduction of 4-nitroanisole using sodium sulfide or hydrogen in the presence of precious-metal catalysts or Raney nickel.
4-Ethoxyaniline

4-Ethoxyaniline has the molecular formula C8H11NO and a molecular weight of 137.18 g/mol, presents a melting point of 2.4 °C and a boiling point of 249.9 °C at a pressure of 101.3 kPa.
At room temperature when pure, it appears colorless, but it turns brown when exposed to light or air. 4-Ethoxyaniline possesses slight solubility in water.
The industrial synthesis of 4-Ethoxyaniline involves the reduction of 4-nitrophenetole using iron or hydrogen under pressure in the presence of a catalyst.
Alternatively, a method that uses heating of 4-chlorophenetole with aqueous ammonia in the presence of copper(I) oxide at 225 °C, yields 85% of the desired 4-ethoxyaniline.
4-Ethoxyacetanilide
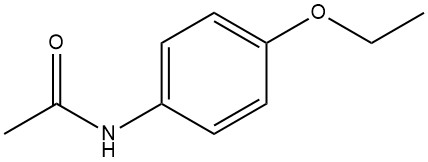
4-Ethoxyacetanilide, commonly referred to as phenacetin, p-acetophenetidine, or 4-acetamidophenetole, has the chemical formula C10H13NO2 with a molecular weight of 179.21 g/mol. This compound is a white crystalline powder and has a melting point within the range of 134 to 135 °C.
It is an odorless compound with a faintly bitter taste. While it has limited solubility in cold water, it is more soluble in hot water, alcohol, ether, and chloroform. At relative humidities between 15 and 90 % the equilibrium moisture content is about 2 % at 25 °C.
The primary method employed for the production of 4-ethoxyacetanilide is the catalytic reduction of 4-nitrophenetole using hydrogen, followed by acetylation with acetic anhydride.
Another synthetic route is the ethylation of 4-nitrophenol using ethyl sulfate in an alkaline environment, subsequent reduction of the nitro group to an amino group by the use of iron in acidic conditions, and final acetylation via boiling with glacial acetic acid.
Alternatively, 4-aminophenol may undergo ethylation using ethyl iodide in an alcoholic alkali solution, yielding 4-phenetidine, which is then subjected to acetylation. The order of reactions can also be reversed, with acetylation preceding ethylation.
Beyond its structural properties, 4-Ethoxyacetanilide possesses both antipyretic and analgesic properties. However, its efficacity is limited in managing severe pain.
Prolonged usage of this product should be exercised with caution due to the potential nephrotoxicity associated with one of its minor metabolites, 2-hydroxyphenetidine, which may contribute to the occurrence of methemoglobinemia.
In laboratory studies using rats, the oral LD50 for 4-Ethoxyacetanilide is reported as 1.65 g/kg.
5-Aminosalicylic Acid
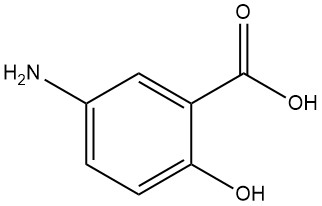
5-Aminosalicylic Acid, also known as 5-amino-2-hydroxybenzoic acid, has the molecular formula C7H7NO3 with a molecular weight of 153.13 g/mol.
It forms a colorless crystals that undergo a transformation to a brown hue when subjected to temperatures exceeding 250 – 260 °C and eventually melting at 283 °C with decomposition.
These crystals are insoluble in ethanol and cold water while shows limited solubility in hot water.
5-Aminosalicylic Acid is produced by the reduction of 3-nitrobenzoic acid at temperatures ranging from 115 to 145 °C and a pressure of 3.5 MPa in a dilute sulfuric acid solution and platinum as catalyst. Under these conditions, the intermediate hydroxylamine derivative undergoes rearrangement, leading to the formation of 5-Aminosalicylic Acid.

Alternatively, another method is the reduction of 5-nitrosalicylic acid, wherein zinc in hydrochloric acid serves as a reducing agent.
N-(4-Hydroxyphenyl)glycine
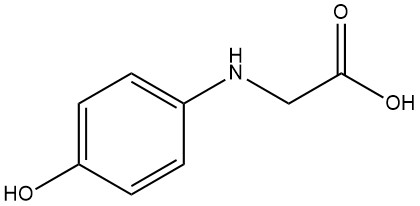
N-(4-Hydroxyphenyl)glycine, also known as 4-hydroxyphenylaminoacetic acid, 4-oxyanilinoacetic acid, or photoglycine has a molecular formula C8H9NO3 and a molecular weight of 167.16 g/mol. It an aggregate spheres or lustrous leaflets when precipitated from water.
Upon heating, its color changes to brown at 200 °C, initiates melting around 220 °C, and eventually completely melts with decomposition within the range of 245 to 247 °C.
N-(4-Hydroxyphenyl)glycine dissolves in alkali and mineral acids, with limited solubility observed in water, glacial acetic acid, ethyl acetate, alcohol, ether, acetone, chloroform, and benzene.
The synthesis of N-(4-Hydroxyphenyl)glycine can be achieved by methods such as reacting 4-aminophenol with chloroacetic acid or by subjecting the corresponding nitrile to alkaline hydrolysis followed by the elimination of ammonia.
N-(4-Hydroxyphenyl)glycine is used as a photographic developer under trade names like Glycine, Iconyl, and Monazol. It also finds application as a photoresist in the dye industry and functions as an intermediate in the production of 4-(N-methylamino)phenol (Metol) through the liberation of CO2.
Furthermore, this compound has utility in analytical chemistry for determining iron, phosphorus, and silicon concentrations, and it serves as an acid indicator in bacteriology.
Prolonged exposure to this compound may lead to kidney damage.
4-Amino-2,6-dichlorophenol

4-Amino-2,6-dichlorophenol has the chemical formula C6H5Cl2NO and a molecular weight of 178.03 g/mol. it can undergo sublimation and melts at 167 °C.
The compound is soluble in ethanol, diethyl ether, acetone, and acetic acid and it has limited solubility in benzene and is nearly insoluble in water.
Reference
- Aminophenols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_099
