2-Butyne-1,4-diol: Properties, Production and Uses

1,4-Butynediol or 2-butyne-1,4-diol is an organic compound that is both an alkyne and a diol with the formula C4H6O2. It is a colorless, hygroscopic solid that is soluble in water and polar organic solvents.
Table of Contents
1. Physical Properties of 1,4-Butynediol
The following table summarizes the physical properties of 1,4-butynediol:
| Property | Value |
|---|---|
| CAS Number | 110-65-6 |
| Formula | HOCH2-C≡C-CH2OH |
| Molecular Weight | 86.09 g/mol |
| Appearance | Colorless, hygroscopic, orthorhombic crystalline solid |
| Melting Point | 58 °C |
| Boiling Point | 150 °C (at 1.8 kPa) |
| Density | 1.2 g/cm³ |
| Solubility in Water | 374 g in 100 g (at 25 °C) |
| Solubility in Polar Solvents (ethanol and acetone) | Soluble |
| Solubility in Ether | Slightly soluble |
| Solubility in Hydrocarbons | Almost insoluble |
| Heat of Vaporization | 50.28 kJ/mol |
| Heat of Combustion | 2204 kJ/mol |
| Flash Point (DIN 51758) | 152 °C |
| Ignition Point | 335 °C |
2. Chemical Properties of 1,4-Butynediol
1,4-Butynediol undergoes relatively slow decomposition between 160 °C and 200 °C. However, violent decomposition can occur at significantly higher temperatures. This decomposition can be initiated or accelerated by various factors:
- Alkaline hydroxide
- Strong anhydrous acids
- Certain heavy-metal salts like Mercury salts and Copper (Cu) and Nickel (Ni) salts (commonly found in hydrogenation catalysts for 2-butyne-1,4-diol)
1,4-Butynediol is sensitive to oxidation. Aqueous solutions of the diol become acidic, likely due to the presence of residual formaldehyde.
Substitution of the hydroxyl groups follows a similar pattern to other diols. Reactions with halogens generate highly reactive products. Notably, reactions with bifunctional compounds only lead to linear polymers.
The carbon-carbon triple bond undergoes typical addition reactions observed in acetylene. While two moles of chlorine react per mole of diol, only one mole each of bromine or iodine reacts.
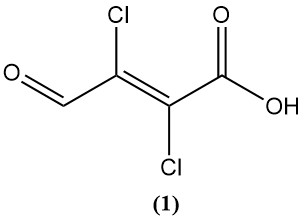
Chlorination in the presence of hydrochloric acid results in the formation of 2,3-dichloro-3-formylacrylic acid (mucochloric acid) (1).
Water addition, catalyzed by mercury salts, yields the relatively unstable 2-oxobutanediol.
3. Production of 1,4-Butynediol
The synthesis of 2-butyne-1,4-diol is based on the work of Reppe et al. and involves the reaction of acetylene and an aqueous formaldehyde solution under pressure, catalyzed by copper acetylide:
2 CH2O + HC≡CH → HOCH2–C≡C–CH2OH (ΔH = −100.5 kJ/mol)
This reversible reaction proceeds through propargyl alcohol, 2-propyn-1-ol, which remains largely on the catalyst and only partially enters the solution. The reaction is first-order with respect to formaldehyde and effectively zero-order with respect to acetylene in industrial processes.
Industrial Process:
The key objective in the synthesis is to minimize the formaldehyde concentration in the resulting butynediol solutions. This is typically achieved by continuous production in cascades of 3-5 reactors.
Two main reactor configurations are employed:
- Fixed-bed: Catalyst is present as strands 3-5 mm in diameter and 10 mm in length. The formaldehyde solution and gaseous acetylene are fed from the top.
- Fluidized-bed: Catalyst is present as particles 0.1-2 mm in diameter. The required amount of acetylene is dissolved in the solution and fed from the bottom, expanding the bed to prevent catalyst loss.
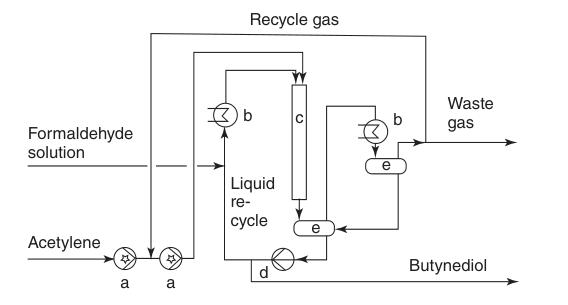
a) Liquid seal pump; b) Cooler; c) Reactor; d) Recycle pump; e) Gas–liquid separator
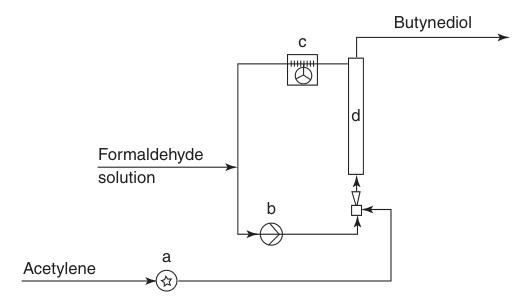
a) Liquid seal pump; b) Recycle pump; c) Air cooler; d) Reactor
Both reactor types utilize liquid-circulation systems to control temperature and ensure optimal conditions.
The catalyst consists of 3-6% bismuth(III) oxide and 10-20% copper(II) oxide, often supported on silica. Copper(II) oxide is converted to copper(I) acetylide by acetylene and formaldehyde, forming the active catalyst. Bismuth oxide inhibits the formation of insoluble polymers known as “cuprenes”.
The Reaction Conditions for this process are:
- Temperature: 80-100°C
- Formaldehyde concentration: 30-50% aqueous solution
- Partial pressure of acetylene: 2-6 bar
- pH: 5-8
The final product solution typically contains:
- 33-55% butynediol
- 1-2% propargyl alcohol
- 0.4-2% unreacted formaldehyde
- 1-2% byproducts (high-boiling materials, sodium formate, methanol)
Butynediol is recovered by vacuum distillation, while propargyl alcohol is recovered in an azeotrope with water.
To avoid handling acetylene above 1.4 bar, suspended catalysts can be used. However, this requires keeping the catalyst in the reactor, often through filtration or recycling, which can be hampered by cuprene formation. Bismuth and malachite-based copper catalysts offer improved performance in this case.
4. Uses of 1,4-Butynediol
Approximately 95% of 2-butyne-1,4-diol is hydrogenated to produce 1,4-butanediol, with some production also going towards 2-butene-1,4-diol.
It is used as an intermediate in the production of the herbicide 3-chloro-2-butynyl N-(3-chlorophenyl)carbamate.
It is also employed as a flame retardant by Solvay through reaction with alkylene oxides and subsequent bromination of the triple bond.
1,4-Butynediol is widely used as a brightening agent in electroplating baths, particularly for nickel and copper.
It acts as a corrosion inhibitor in steel inhibitor pickling processes.
1,4-Butynediol is available commercially in two forms:
- 34% aqueous solution: Traded by Ineos (Golpanol liquid) and BASF (Korantin liquid), with the latter containing added hexamethylenetetramine.
- Anhydrous product: Available as flakes in distilled form (BASF) with 99% purity under various trade names such as Butynediol pure cryst., Korantin solid, and Golpanol pure solid.
5. Toxicology of 2-Butyne-1,4-diol
2-Butyne-1,4-diol is classified as toxic because of the following data:
- LD50 value: The LD50 value for 2-butyne-1,4-diol is about 100mg/kg (rat, oral). This falls within the range of 50-200mg/kg, which corresponds to “highly toxic” according to standard classifications.
- Skin and eye irritation: The compound causes strong irritation to the skin, especially after prolonged contact. It also irritates the eyes.
- Inhalation: While no adverse effects were observed in rats exposed to saturated air for 8 hours, this does not necessarily indicate complete safety. Further studies are needed to understand the potential inhalation hazards.
The absence of established TLV and MAK values for 2-butyne-1,4-diol further emphasizes the need for caution. These values represent occupational exposure limits and their absence suggests a lack of sufficient data to determine safe exposure levels.
Reference
- Butanediols, Butenediol, and Butynediol; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_455.pub2
