Malic Acid: Properties, Production and Uses
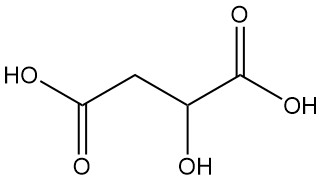
What is Malic Acid?
Malic acid, also known as hydroxysuccinic acid or hydroxybutanedioic acid, is a dicarboxylic acid with the chemical formula C4H6O5. It is a naturally occurring organic compound characterized by a tart taste.
It was first described by Sheele who, in 1785, isolated this acid from unripe apples. The name malic is from the Latin for apple, malum.
Malic acid is a constituent of various fruits, notably apples. In biological systems, malic acid, in its ionized form, malate, serves as an intermediate in the tricarboxylic acid cycle. This metabolic pathway is crucial for energy production.
The L-isomer of malic acid is the predominant form found in nature and possesses biological activity. While primarily known for its role in food flavoring, emerging evidence suggests potential benefits in human health.
Malic acid is involved in energy metabolism, particularly in conditions of hypoxia. Studies indicate its potential to enhance physical performance and alleviate symptoms associated with fibromyalgia.
Malic acid metabolism is influenced by enzymes such as malic enzyme. This enzyme catalyzes the oxidative decarboxylation of malate to pyruvate. The generated pyruvate can undergo further metabolic transformations, including conversion to glucose or oxaloacetate.
Malic acid shows antimicrobial properties. Its acidic nature contributes to food preservation by inhibiting microbial growth. However, its effectiveness is influenced by factors such as pH and specific microbial species.
Table of Contents
| Property | Value |
|---|---|
| CAS Number | [617-48-1] |
| Chemical Formula | C4H6O5 |
| Molecular Mass | 134.09 g/mol |
| Melting Point | 131 °C |
| Density | 1.60 g/cm3 |
| Viscosity of 50% aqueous solution at 25 °C | 6.5 mPa.s |
| Heat of Combustion at 20 °C | -1.340 MJ/mol |
| pKa1 at 20 °C | 3.51 |
| pKa2 at 20 °C | 5.03 |
| Property | Value |
|---|---|
| CAS Number | [97-67-6] |
| Melting Point | 100 °C |
| Density at 20 °C | 1.595 g/cm3 |
| Specific rotation at 18 °C (7 wt % in H2O) | -2.3° |
| Solubility in water at 20 °C | 36.4 g/100 g |
2. Chemical Reactions of Malic Acid
Due to the presence of carboxylic acid and hydroxyl functional groups in malic acid, it is capable of undergoing a variety of chemical reactions.
As a carboxylic acid, malic acid reacts with bases to form salts, such as sodium malate or potassium malate.
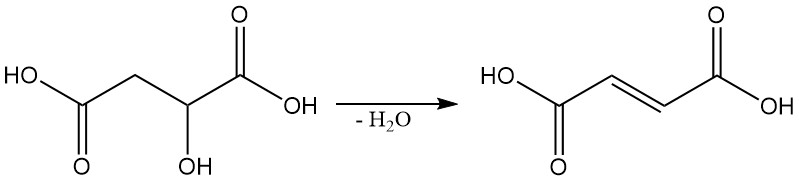
Under specific conditions, malic acid can be dehydrated to produce fumaric acid.
The decarboxylation of malic acid yields pyruvic acid.
Malic acid can react with alcohols to form esters, which are often used as flavoring agents.
Malic acid can be oxidized to produce various products, such as oxaloacetic acid, oxalic acid, and carbon dioxide, depending on the conditions.
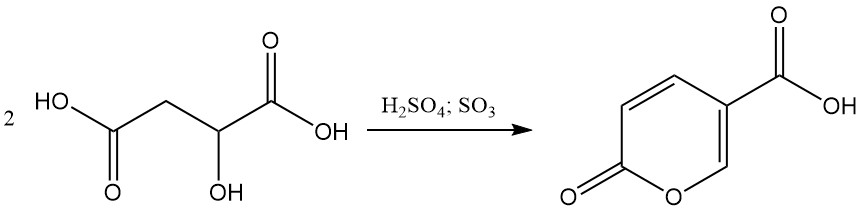
In the presence of 20%–30% fuming sulfuric acid, malic acid undergoes self-condensation to form coumalic acid.
Malic acid can form complexes with metal ions such as calcium and magnesium.
Malolactic fermentation is a common process in winemaking where malic acid is converted into lactic acid by bacteria. it is also an intermediate in the Krebs cycle, in which it undergoes oxidation to oxaloacetic acid.
3. Production of Malic Acid
3.1. Chemical Production of Malic Acid
Malic acid synthesis primarily relies on chemical processes, yielding racemic D,L-malic acid. The precursor, maleic anhydride, is derived from fossil hydrocarbons, mainly n-butane. Benzene, a former raw material, remains in use, particularly in Asian countries.
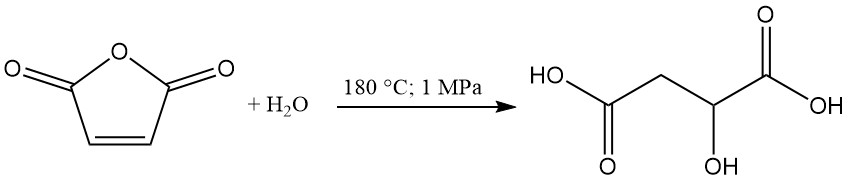
(R,S)-Malic acid is produced industrially in the United States and Canada by maleic anhydride hydration. Alberta Gas is the sole US manufacturer with a production capacity of approximately 5000 metric tons annually.
In this process, maleic anhydride is heated at 180 °C under 1 MPa pressure to form malic acid as the primary product. Maleic acid and fumaric acid are generated as byproducts. Fumaric acid, due to its low water solubility, is separable by filtration and recycled.
Subsequent concentration of the filtrate gives malic acid. The crude acid is purified by repeated washing, evaporation, and recrystallization to reduce fumaric and maleic acid impurities to 7.5 and <500 ppm, respectively.
To obtain pharmaceutical-grade malic acid, additional purification steps are necessary.
3.2. Enzymatic L-Malic Acid Production
Enantiopure L-malic acid, which is preferred for pharmaceuticals and polymers, is challenging to obtain via racemic D,L-malic acid resolution. Enzymatic synthesis offers a selective and milder alternative.
Fumarase-catalyzed hydration of fumaric acid to L-malic acid is the principal enzymatic process. This can be achieved using purified enzymes, permeabilized or lyophilized cells, or whole cells.
Microorganisms like Saccharomyces cerevisiae, Brevibacterium flavus, Brevibacterium ammoniagenes, and Rhizopus oryzae show high conversion rates between 80% and ≈100% in whole-cell catalysis. Immobilization makes the process more economic by allowing the catalyst to be reusable.
3.3. Biosynthesis of Malic Acid by Microorganisms
Microbial malic acid production offers advantages over chemical synthesis, including exclusive L-malic acid formation and the use of diverse, renewable substrates.
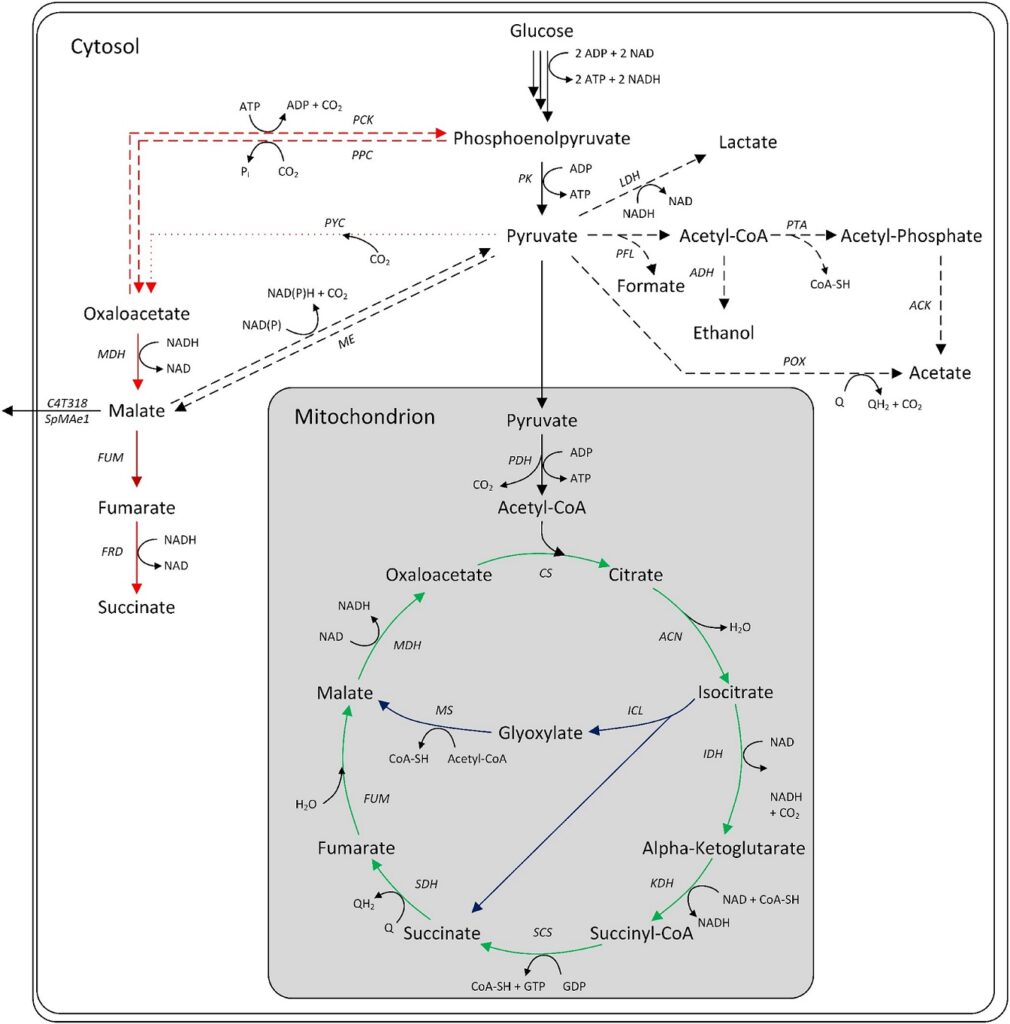
Three primary intracellular pathways for microbial malic acid production exist: oxidative tricarboxylic acid (TCA) cycle, reductive TCA (rTCA) cycle, and glyoxylate cycle. The oxidative TCA, located in the mitochondrion, converts acetyl-CoA to citrate, which is subsequently oxidized to L-malate with carbon dioxide loss.
The maximum theoretical yield is 1 mol/mol. Some Aspergillus species possess cytosolic TCA isoforms, enabling a reductive TCA pathway. This ATP-neutral route carboxylates pyruvate to oxaloacetate, which is converted to L-malate by malate dehydrogenase. A theoretical yield of 2 mol/mol glucose is possible.
The glyoxylate cycle converts citrate to succinate and glyoxylate, with subsequent condensation to L-malate. The maximum yield is 1 mol/mol, which can increase to 1.33 mol/mol if the oxaloacetate consumed is replenished by the carboxylation of pyruvate.
Microorganisms used in L-malic acid production include natural microorganisms (such as Aspergillus flavus, Aspergillus oryzae, Ustilago trichophora, and Rhizopus delemar) and genetically modified fungi and bacteria.
4. Uses of Malic Acid
(R,S)-Malic acid shares physicochemical properties with citric acid and tartaric acid but possesses a milder flavor profile. This characteristic favors its application in food products where citric acid’s acidity is undesirable. Examples include food packaging and baking.
In the food industry, malic acid is widely used (about 85–90%) as an acidulant and acidity regulator. Its flavor differs from that of citric acid, offering a less intense but longer-lasting sourness. This property aids in masking artificial sweetener aftertaste and creating balanced flavor profiles in beverages.
Synergistic interactions between malic acid and sweeteners enable a sweetener reduction of up to 20% and potential cost savings. The use of anhydrous malic acid powder offers additional economic advantages.
Primary applications include confectionery, jams, jellies, and canned fruits and vegetables. Food additive regulations in most countries allow its use.
Enantiomeric malic acids, (R)-(+)-malic acid and (S)-(-)-malic acid, can be obtained from (R,S)-malic acid resolution or by microbial fermentation of fumaric acid.
Beyond the food industry, malic acid is used as a buffer and chelating agent in personal care and cleaning products. Pharmaceutical applications include its use as a drug component, while semiconductor manufacturing uses it in polishing and cleaning processes.
Additionally, malic acid is employed in animal feed and as a component in low-transition-temperature mixtures.
Malic acid is a dicarboxylic acid, which enables its use as a polymer building block. Copolymers and homopolymers of malic acid exhibit properties such as hydrophilicity, biocompatibility, and biodegradability, finding potential applications in fields like drug delivery and materials science.
The main manufacturers of malic acid include Bartek (Canada), the Japanese companies Fuso Chemical and Mitsubishi Corporation Life Sciences, Isegen (South Africa), Polynt (Italy), Thirumalai Chemicals (India), the Chinese companies Changmao Biochemical Engineering Company, Anhui Sealong Biotechnology, and Jinhu Lile Biotechnology Industry, as well as Tate & Lyle (UK) and Yongsan Chemicals (Korea).
5. Toxicology of Malic Acid
Malic acid shows low acute toxicity in animal tests. Oral LD50 values range from 1.6 to 5 g/kg across species. Intravenous and intraperitoneal LD50 values are significantly lower.
Chronic oral studies in rats and dogs revealed minimal adverse effects, primarily including minor alterations in body weight and feed consumption. Reproductive toxicity studies yielded negative results.
Dermatological studies indicate moderate skin irritation in rabbits and severe ocular irritation in rabbits. Malic acid is a strong irritant to guinea pig skin.
Mutagenicity assessments yielded inconsistent results. Malic acid itself was non-mutagenic in various assays, but its pyrolysates and chlorinated derivatives showed mutagenic properties.
Skin irritation studies reported dose-dependent effects, with higher pH correlating to increased irritation. Predictive testing in atopic dermatitis patients indicated potential skin reactivity to malic acid-rich diets. Malic acid also influenced cell renewal rates depending on a pH values.
Clinical efficacy and safety trials reported no toxicity.
Chronic toxicity studies on malic acid in rats and dogs established No Observed Effect Levels (NOEL) of 5000 ppm and 50000 ppm, respectively.
Fumaric acid chronic toxicity data in rats indicate a No Observed Adverse Effect Level (NOAEL) of approximately 600 mg/kg body weight/day. Given fumaric acid’s metabolic conversion to malic acid within the Krebs cycle, cross-species extrapolation is considered valid.
Malic acid exposure assessment from dietary concentration to mg/kg/day yielded uncertain results. Estimated ranges varied between 2–200 mg/kg/day and 25–2500 mg/kg/day. Applying EFSA conversion factors addressed this uncertainty, resulting in a NOAEL of 260 mg/kg/day.
References
- Hydroxycarboxylic Acids, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_507
- https://scijournals.onlinelibrary.wiley.com/doi/10.1002/jctb.6269
- https://pubmed.ncbi.nlm.nih.gov/11358110/
- https://onlinelibrary.wiley.com/doi/10.1002/0471743984.vse9535
- https://onlinelibrary.wiley.com/doi/10.1002/9780470995327.ch157
- https://echa.europa.eu/registration-dossier/-/registered-dossier/11511/7/6/1
- https://www.orgsyn.org/demo.aspx?prep=cv4p0201
