Chloroacetaldehyde: Properties, Production and Uses

Chloroacetaldehyde [107-20-0], also known as 2-chloroethanal, is an organic compound with the formula CH2ClCHO. It is a colorless liquid that was first produced in pure form by K. Natterer in 1882 by heating chloroacetaldehyde diethyl acetal with anhydrous oxalic acid at 100–150 °C.
Table of Contents
1. Physical Properties of Chloroacetaldehyde
1.1. Anhydrous Chloroacetaldehyde
Anhydrous chloroacetaldehyde is a colorless, mobile liquid with a pungent odor that has the following physical properties:
- Molecular weight: 78.50 g/mol
- Boiling point: 85–85.5°C (99.7 kPa)
- Enthalpy of formation: -256.4 kJ/mol
- Enthalpy of combustion: -981.4 kJ/mol (101.3 kPa, 20°C)
- Dipole moment: 1.99 D (298 K, benzene)
- Soluble in water (hydrate formation) and common organic solvents
- Octanol-water partition coefficient: 0.16 (very low)
1.2. Monochloroacetaldehyde Hemihydrate
- Formula: CH2ClCH(OH)2OCH(OH)CH2Cl (1,1′-dihydroxy-2,2′-dichlorodiethyl ether)
- Molecular weight: 175.01 g/mol
- Colorless crystals with melting point between 43 and 50 °C (dehydration)
- Decomposes at the boiling point (84 °C)
- Soluble in water, ethanol, ether, benzene, dichloromethane, and chloroform.
- CAS numbers [34789-09-8] (“acetaldehyde chloro dimer hydrate”) and [7737-02-2] (“1,1′-oxybis(2-chloroethanol)”) refer to the same compound.
- Forms an azeotropic mixture with water, inseparable by simple distillation.
- Flash point: 60°C
- Ignition point: 405°C
- Explosive limits in air (20°C, 101.3 kPa): lower 305 g/m³
| Temperature (°C) | Solubility (wt%) |
|---|---|
| 1 | 13.35 |
| 10 | 22.2 |
| 20 | 44.3 |
| 30 | 62.7 |
| 40 | 81.5 |
| Concentration (wt%) | Density (g/cm³) |
|---|---|
| 10 | 1.041 |
| 20 | 1.085 |
| 30 | 1.137 |
| 40 | 1.188 |
| 50 | 1.238 |
| 60 | 1.290 |
| 72.5 | 1.355 |
2. Chemical Properties of Chloroacetaldehyde
Chloroacetaldehyde is very reactive due to both the aldehyde and chloromethyl groups. Its α-hydrogens and chlorine are activated, facilitating nucleophilic substitutions and reactions typical of aldehydes (addition, condensation).
2.1. Reactions of the aldehyde group
Self-condensation of chloroacetaldehyde at room temperature forms cyclic trimers, tetramers, and polyoxymethylene polymers. Cotrimers and copolymers with acetaldehyde also exist. The hemihydrate 1,1′-dihydroxy-2,2′-dichlorodiethyl ether structure reacts similarly to an aldehyde.
Sodium bisulfite adduct and dimethyl/diethyl acetals are important derivatives of chloroacetaldehyde used as pharmaceutical and pesticide precursors.
Oxidation of chloroacetaldehyde with air/cobalt salts, hydrogen peroxide, or nitric acid yields monochloroacetic acid.

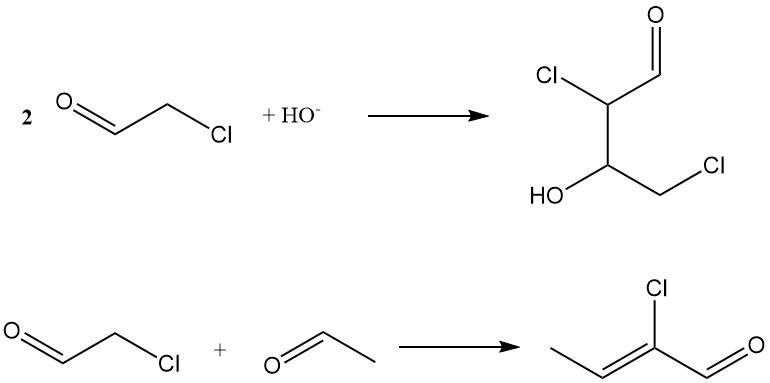
The aldol condensation in an alkaline medium produces 2,4-dichloroacetaldol, and the coaldolization with acetaldehyde forms 2-chlorocrotonaldehyde, which is also a byproduct of acetaldehyde production.
Other reactions include the formation of aldehyde ammonia, cyanohydrin, and 2-chloroethyl chloroacetate (Tishchenko reaction).
2.2. Reactions of the chlorine atom
Hydrogenation of chloroacetaldehyde in an aqueous solution with a palladium-carbon catalyst removes the chlorine. Various nucleophiles can replace the chlorine.
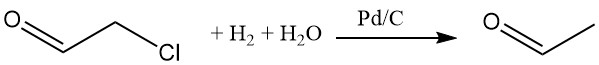
Chloroacetaldehyde reacts with sodium mercaptan to produce mercaptoacetaldehyde, which is used as an intermediate in the synthesis of thienodiazepines, azo dyes, d-l-cystein, and certain food flavors.

Amino compounds can substitute the chlorine atom of chloroacetaldehyde in acetal form (avoid reactions with the aldehyd group). These intermediates are used in the synthesis of pesticides and pharmaceuticals.

Chloroacetaldehyde dimethyl acetal reacts with sodium methoxide to form 1,1,2-trimethoxyethane (a pharmaceutical intermediate).

2.3. Combined Reactions
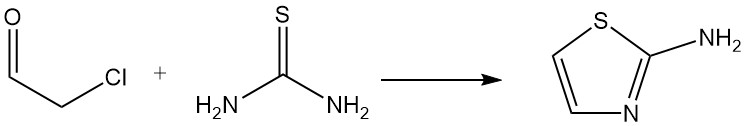
Chloroacetaldehyde (hemihydrate or acetal) is an important starting material for the synthesis of heterocycles; one example is the reaction with thiourea to form 2-aminothiazole, which is used in the production of pharmaceuticals and dyes.
The cyclocondensation with methyl acetoacetate gives 2-methyl-3-carbomethoxyfuran (a seed disinfectant).
The reaction of chloroacetaldehyde with carboethoxyhydrazine, followed by cyclization with thionyl chloride, produces 5-chlorothiadiazole, a cotton defoliant and plant growth regulator.
Stilbenes are formed by reactions with aromatic hydrocarbons, phenols, or phenol ethers. Vinyl dialkyl phosphates are produced by the Perkow reaction with trialkyl phosphites. 1,1,2-Triacetoxyethane (d,l-serine precursor) is prepared by reacting chloroacetaldehyde with sodium acetate and acetic anhydride.
2.4. Chloroacetaldehyde Polymers
1. Trichloroparaldehyde (C6H9Cl3O3)
- Properties: colorless crystals, mp 88–89 °C, bp 140–144 °C, soluble in common organic solvents.
- Formation:
- Reaction of concentrated sulfuric acid with chloroacetaldehyde hemihydrate.
- Residue from the azeotropic distillation of chloroacetaldehyde hemihydrate.
- Decomposition of 1,2-dichloroethyl nitrate in the presence of Lewis acids.
It is converted to chloroacetaldehyde on heating with acids.
2. Tetrachlorometaldehyde (C8H12Cl4O4)
- Properties: colorless crystals, mp 65–67 °C, bp 127–130 °C.
- Formation: Residue from azeotropic dehydration of chloroacetaldehyde hemihydrate.
3. Polychloroacetaldehydes (C2H3ClO)n
- Properties: amorphous, elastomeric products with polyacetal structure. Crystalline forms available at lower temperatures with specific catalysts.
- Formation: polymerization of anhydrous chloroacetaldehyde at -40 to -78°C, especially with Lewis acids.
3. Production of Chloroacetaldehyde

Chloroacetaldehyde is produced by the reaction of acetaldehyde or paraldehyde with chlorine, with dichloroacetaldehyde and trichloroacetaldehyde as impurities. These tree products are also obtained as byproducts in the Wacker process for acetaldehyde production.
Another production method is the chlorination of vinyl chloride in water at 20 °C, which yields nearly a 100% chloroacetaldehyde yield if its concentration in the reactional medium stays below 5%. Higher concentrations favor 1,1,2-trichloroethane formation.
The reaction of chlorine with vinyl acetate in water at room temperature forms a concentrated chloroacetaldehyde solution, which can be distilled to produce the pure compound. Side products are minor amounts of chlorinated acetaldehydes, acetaldehyde, and condensation products.
Synthesis of chloroacetaldehyde acetals with high yields and purity is achieved by conducting the above reactions in alcohols.
Production of anhydrous chloroacetaldehyde by chlorination of dry acetaldehyde or paraldehyde gives a low yield. However, azeotropic dehydration of the hemihydrate with chloroform, toluene, or carbon tetrachloride followed by distillation over a dehydrating agent offers better results.
Anhydrous chloroacetaldehyde is obtained with high yields by depolymerization of trichloroparaldehyde or polychloroacetaldehyde at 145 °C with oxalic or trichloroacetic acid. Pyrolysis of chloroethylene carbonate in the presence of quaternary ammonium salts also gives the same result.
4. Uses of Chloroacetaldehyde
Chloroacetaldehyde is used as a building block in the production of intermediates for statin drugs, which lower cholesterol levels, and for anti-AIDS drugs. The mercapto derivative, 2,5-dihydroxy-1,4-dithiane, is used in the synthesis of medications against HIV/AIDS.
Chloroacetaldehyde is also used in the synthesis of various dyestuffs. Its dialkylacetal derivatives, like chloroacetaldehyde dimethyl acetal, are important starting materials for the production of agricultural chemicals and its acetals are utilized in the production of fragrances.
It can form polymers, which are commercially not important.
5. Toxicology of Chloroacetaldehyde
Acute Toxicity:
- Highly toxic via inhalation, causing rapid to delayed death in animals. Lethal dose (LD50) values are:
- Rat (oral): 89 mg/kg
- Rabbit (dermal): 267 mg/kg
- Rat (inhalation, 1h): 650 mg/m³
- Skin and eye irritant, even at low concentrations (0.03% solution).
- Not a skin sensitizer in guinea pigs.
Chronic Toxicity:
- No signs of toxicity were observed in chronic inhalation studies below 5.2 mg/m³.
- Mutagenic in various organisms and human cells.
- No skin tumor increase was observed in mouse studies.
- Liver tumors were observed in male mice drinking water with 0.1 g/L chloroacetaldehyde.
Human Exposure:
- Chloroacetaldehyde is a skin, mucous membrane, and respiratory tract irritant.
- It causes burn-like blisters and tissue damage on skin contact.
- Lacrimation and nasal irritation at 32.6 mg/m³ air exposure.
Recommendations:
- TLV-STEL (USA): 1 ppm (maximum allowed air concentration).
- EU classification: “Very Toxic” and “Dangerous to the Environment.”
- Requires full personal protective equipment (goggles, gloves, and mask).
Reference
- Chloroacetaldehydes; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_527.pub2
