Benzidine: Properties, Production and Uses
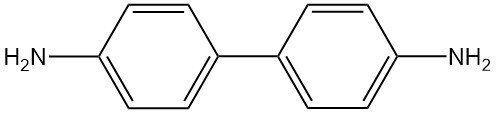
Benzidine is an organic compound with the chemical formula C12H12N2. It is a rayish-yellow, reddish-gray, or white crystalline solid that is slightly soluble in water.
Benzidine is a diphenyl bases that is widely used as intermediates in the manufacture of azo dyes and pigments. It is also used as cross-linking agents in polyurethane plastics and as analytical and diagnostic reagents.
Benzidine can react with a variety of substances, including cations, anions, organic compounds, and oxidizing agents. This versatility makes them valuable tools for a wide range of applications.
Table of Contents
1. Physical Properties of Benzidine
Benzidine is a diacidic compound with the chemical formula C12H12N2 and the molar mass of 184.24 g/mol. The physical properties of benzidine are listed in the following table:
| Property | Value |
|---|---|
| Appearance | White powder |
| Melting point | 128 °C (coarse rods), 122-125 °C (metastable modification) |
| Boiling point | 400-401 °C |
| Density | 1.25 g/cm³ |
| Solubility in water | 1 part by weight in 2447 parts of water at 12 °C, 106.5 parts of water at 100 °C |
| Solubility in organic solvents | Sparingly soluble in ether (45 parts) and absolute ethanol (13 parts) at 20 °C |
| Acidity | Diacidic (can donate two protons in solution) |
| Dissociation constants (at 30 °C) | K1 = 9.3 x 10-10, K2 = 5.6 x 10-11 |
| Heat of neutralization | 106.5 kJ/mol |
| Vapor pressure | < 0.1 mmHg at 25 °C |
| Refractive index | 1.652 at 25 °C |
| Flash point | 205 °C |
| Autoignition temperature | 300 °C |
2. Chemical Reactions of Benzidine
Benzidine exhibits the following chemical reactions:
- Air exposure: Benzidine discolors when exposed to air.
- Water resistance: Benzidine is resistant to water.
- Color reactions: Benzidine forms blue, green, or red colorations and precipitates with oxidizing agents. These reactions are valuable for detecting oxidizing agents.
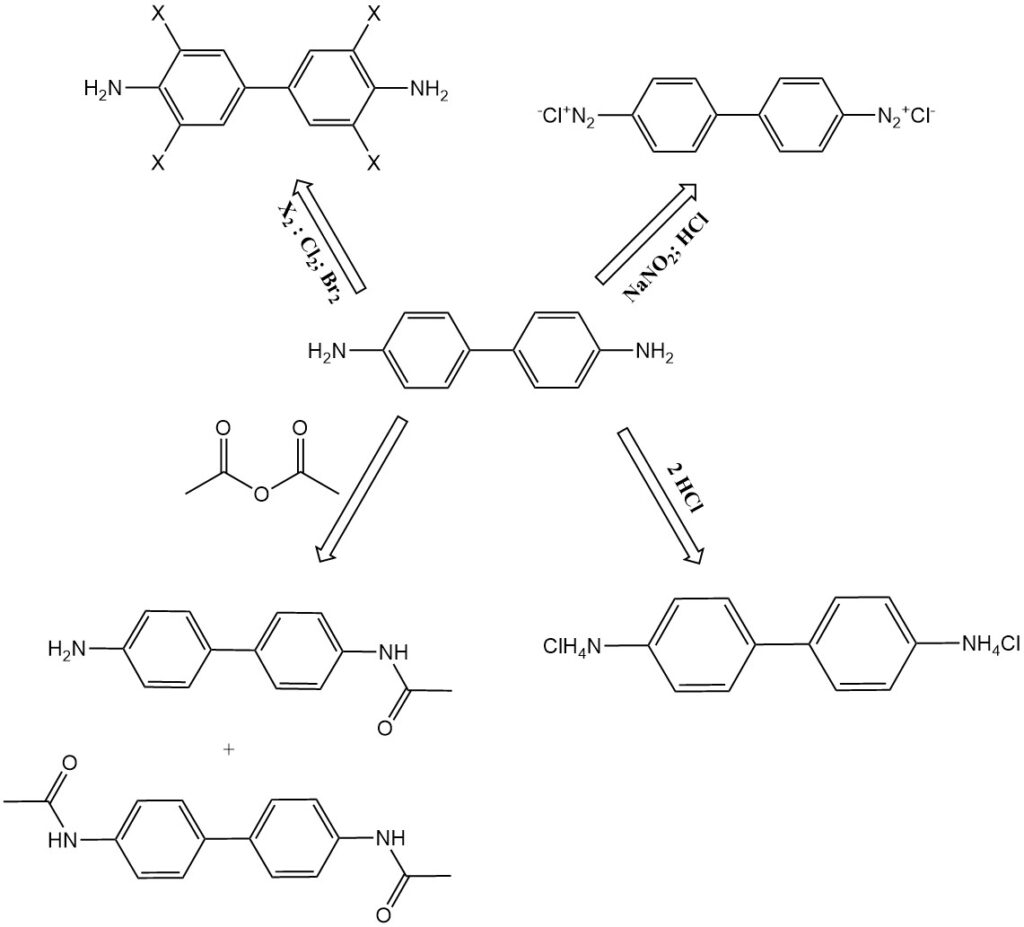
- Chlorination and bromination: Benzidine transforms into 3,3′,5,5′-tetrachlorobenzidine and tetrabromobenzidine, respectively.
- Nitration and sulfonation: Benzidine sulfate yields 2-nitrobenzidine, 2,2′-dinitrobenzidine, and 2,3′-dinitrobenzene by nitration reactions. Sulfonic acids and sulfones of benzidine can be formed under sulfonating conditions.
- N-acetylation: Benzidine reacts with acetic anhydride to form N-acetylation products, such as N-acetyl-benzidine and N,N’-diacetyl-benzidine. These compounds are also found as metabolites in animal digestion.
- Diazotization: Benzidine can be diazotized to form tetrazonium compounds. The first diazonium group couples vigorously, while the second reacts more slowly. This enables the production of asymmetrical diazo dyes.
- Salt formation: Benzidine forms salts with acids, such as benzidine monohydrochloride, benzidine dihydrochloride, and benzidine sulfate.
3. Production of Benzidine
Benzidine is produced in three stages:
- Nitro group reduction to form hydrazo compounds
- Benzidine rearrangement
- Base isolation
Benzidine has been industrially manufactured from nitrobenzene since the late 19th century. Common production methods include alkaline iron reduction, amalgam reduction, and electrochemical reduction.
The resulting hydrazobenzene is rearranged with hydrochloric acid or sulfuric acid during cooling, and the product is isolated as benzidine hydrochloride or benzidine sulfate. To minimize the risk of chronic toxicity, conversion of these salts to the free base is avoided whenever possible.
3.1. Reduction of Nitrobenzene
The reduction of nitrobenzene to hydrazobenzene can represented by the following equation:
2 C6H5NO2 + 10 H → C6H5NH-NHC6H5 + 4 H2O
This reduction process is known as the Haber process and progresses through several stages, involving the intermediate condensation of a nitroso compound with a hydroxylamine compound to form the azoxy compound.
This method results in the formation of symmetrical hydrazo compounds. The primary byproduct generated is the monocyclic primary amine, such as aniline, corresponding to the original nitro compound. This byproduct is formed from both the further reduction of the phenylhydroxyamine and the disproportionation of the unstable hydrazo compound.
Various reduction techniques are employed in industrial applications, including:
1. Reduction with Zinc Dust: This method involves the reduction of aromatic nitro compounds with zinc dust in an alkaline environment. The reaction is given by:
2 C6H5NO2 + 5 Zn + 10 NaOH → C6H5NH-NHC6H5 + 5 Na2ZnO2 + 4 H2O
Industrially, the nitrobenzene is dissolved in a high-boiling solvent, and zinc dust is suspended in the solution. Sodium hydroxide solution is emulsified, and the reduction takes place. Careful control of the reaction temperature is necessary, and the process is completed when the azo stage’s red color vanishes.
2. Reduction with Iron: Reduction with iron and sodium hydroxide solution is less common in preparative chemistry but has been explored for commercial production. The reduction equation is:
2 C6H5NO2 + 4 Fe + 6 H2O → C6H5NH-NHC6H5 + 2 Fe(OH)2 + 2 Fe(OH)3
Various forms of iron, such as cast iron turnings or iron powder, are used in this method. The reduction is conducted in a molar ratio of 1:2:4 for nitrobenzene, iron, and sodium hydroxide, respectively.
3. Reduction with Sodium Amalgam: Sodium amalgam is employed for the reduction of nitrobenzene at elevated temperatures. The reduction occurs in an emulsified mixture of nitrobenzene in water or sodium hydroxide, and azobenzene product is formed. However, some overreduction to monocyclic amines may occur during this process.
4. Electrolytic Reduction: Commercial-scale electrochemical reduction is another method used for the reduction of nitro to hydrazo compounds. It involves the use of electrolytic cells with cathodes, diaphragms, and anodes, and the reaction can be represented as:
2 C6H5NO2 + 10 H+ + 10 e– → C6H5NH-NHC6H5 + 4 H2O
The reduction is conducted at elevated temperatures and specific current densities, with the process being regulated by controlling the addition of reactants.
5. Catalytic Reduction: Nitrobenzene can be reduced to hydrazobenzene by catalytic hydrogenation in the presence of a palladium–carbon catalyst. This method may include dilute alcohol, a base, and elevated temperature and pressure conditions.
There are other reducing agents used in preparative chemistry, such as various metals, amalgams, and organic reducing agents like methanol, formaldehyde, and glucose, which are employed based on specific reaction requirements.
3.2. Benzidine Rearrangement
The benzidine rearrangement is a key step in producing diaminodiphenyl compounds. These compounds are formed by rearranging aromatic hydrazo compounds, which are initially derived from reducing aromatic nitro compounds with an alkaline solution.
The rearrangement process, typically catalyzed by mineral acids, leads to the formation of diaminodiphenyl compounds (1), (2) and (3) and aminodiphenylamine compounds (4) and (5).

- Ortho rearrangements produce small amounts of o-benzidine (2,2′-diaminodiphenyl) (2) and diphenyline (2,4′-diaminodiphenyl) (3).
- Partial rearrangements produce o-semidine (2-aminodiphenylamine) (4) and p-semidine (4-aminodiphenylamine) (5).

The type and quantity of rearrangement products are influenced by the starting material’s chemical structure but can only be minimally adjusted by varying reaction conditions. Some byproducts, such as diphenyline (3), are produced in quantities of up to 15% but have no commercial value.
The benzidine rearrangement is a true intramolecular reaction, meaning it doesn’t yield mixed benzidines from mixtures of different hydrazo compounds. Instead, it exclusively produces the corresponding unsymmetrical benzidine from unsymmetrically substituted hydrazobenzenes.
In industrial process, the benzidine rearrangement begins with the hot solution obtained from reducing the nitrobenzene to the hydrazobenzene. While intermediate isolation of the hydrazobenzene is not always necessary, it is advisable in specific cases, such as amalgam reductions.
To reduce the azo content of the hydrazobenzene, some reducing agents like sodium hydrosulfite (sodium dithionite) or zinc dust can be added before the rearrangement.
The reaction occurs when the hydrazobenzene solution is mixed with a suitable mineral acid, typically 10 – 30% hydrochloric acid, 20 – 80% sulfuric acid, or a mixture of the two.
The optimum temperature for the rearrangement is 100 °C for the diphenyl bases. It is crucial to maintain temperatures within the appropriate range, as excessive heat can reduce the yield, which is typically targeted at 70 – 95%.
3.3. Isolation of Pure Product
The most important stage of the benzidine rearrangement is the formation of the hydrochloric or sulfuric acid salt of benzidine. This salt can be either isolated directly, such as by salting out with sodium chloride or sodium sulfate, or first converted into the free base using a dilute alkali, such as sodium hydroxide solution or ammonia solution.
The byproducts, especially the aniline and diphenyline, can be separated out due to their greater solubility. The azobenzene is the only compound remaining in the inert solvent after acid extraction and can be returned to the reduction process.
4. Uses of Benzidine
Benzidine has several applications, including:
- Dye production: Benzidine is used to produce azo dyes for wool, cotton, and leather. However, its use in this capacity has declined due to its carcinogenicity.
- Quantitative determination of sulfuric acid: Benzidine can be used to determine the concentration of sulfuric acid.
- Detection and determination of anions and metal ions: Benzidine can be used to detect and quantify various anions and metal ions.
- Detection of free chlorine or pyridine: Benzidine can be used to detect trace amounts of free chlorine or pyridine in drinking water.
- Blood detection: Benzidine can be used to detect blood based on its color change from green to blue in the presence of hydrogen peroxide and peroxidases.
- Rubber production: Benzidine is used as a cross-linking agent in the production of rubber products, such as tires and hoses.
- Plastic production: Benzidine is used as a stabilizer in the production of some plastics.
- Pharmaceutical production: Benzidine is used as an intermediate in the production of some pharmaceuticals, such as antihistamines and antispasmodics.
Benzidine is a highly toxic compound, but it continues to be used in various chemical syntheses and applications.
5. Toxicology of Benzidine
Acute oral toxicity: LD50 in rats = 1.57 g/kg
Subacute dietary exposure: Adverse effects in mice include cloudy swelling of the liver, vacuolar degeneration of renal tubules, hyperplasia of myeloid elements in the bone marrow, and lymphoid cell changes in the thymus and spleen.
Dermal and pulmonary absorption: Limited information is available, but systemic manifestations of toxicity suggest significant absorption may occur.
Intravenous injection and metabolism: Benzidine is converted into N-acetyl-benzidine and N,N’-diacetylbenzidine, which are further transformed into N-hydroxy-N,N’-diacetylbenzidine and 3-hydroxy-N,N’-diacetylbenzidine. The latter compound binds to nucleic acids.
Ames test results: Benzidine tests positive in the Ames test, and its metabolites have also been shown to induce mutations.
DNA effects: Benzidine leads to DNA strand breaks and cell transformation. It induces unscheduled DNA synthesis in HeLa cells and rat hepatocytes.
Carcinogenic properties: Benzidine hydrochloride has been shown to induce hepatocellular carcinoma in mice and other cancers in rats, hamsters, and humans.
Benzidine is a potent carcinogen in animals and humans. It is classified as Group A1 by the MAK commission and Group A1b by the ACGIH.
Reference
- Benzidine and Benzidine Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_539
