Benzaldehyde Condensation
Key Takeaways
- Benzaldehyde condensation is a versatile chemical reaction that can be used to synthesize a wide range of organic compounds.
- The reaction is typically catalyzed by a strong base, such as sodium hydroxide or potassium hydroxide.
- The mechanism of benzaldehyde condensation involves the formation of an enolate anion, which then reacts with another aldehyde or ketone to form an α-orβ-hydroxy aldehyde or ketone.
- Benzaldehyde condensation can be used to synthesize a variety of important compounds, such as benzoin, cinnamic acid, cinnamaldehyde, and heterocyclic compounds.
- The reaction has a wide range of applications in the pharmaceutical, food, and chemical industries.
Table of Contents
1. What is Benzaldehyde Condensation?
Benzaldehyde condensation is a chemical reaction between benzaldehyde and an aldehyde or ketone molecules with an α-hydrogen to form an β-hydroxy aldehyde or ketone. The reaction is typically catalyzed by a strong base, such as sodium hydroxide or potassium hydroxide.
There are two main types of benzaldehyde condensation:
- Benzoin condensation: This type of condensation reaction occurs between two aldehyde molecules in the presence of a cyanide ion catalyst. The product of the benzoin condensation is an α-hydroxy ketone.
- Aldol condensation: This type of condensation reaction occurs between an aldehyde and a ketone, or between two aldehydes, in the presence of a base catalyst. The product of the aldol condensation is a β-hydroxy aldehyde or ketone.
The general equation for benzaldehyde condensation is as follows:

2. History of Benzaldehyde Condensation
Benzaldehyde condensation was first discovered in 1832 by Justus von Liebig and Friedrich Wöhler. They observed that the reaction of benzaldehyde with potassium cyanide produced a new compound, which they named benzoin.
In the late 1830s, Nikolay Zinin developed a catalytic version of the benzoin condensation using sodium cyanide. This made the reaction more practical and accessible to chemists.
Since then, benzaldehyde condensation has become one of the most important and widely used carbon-carbon bond formation reactions in organic chemistry.
3. Mechanism of Benzaldehyde Condensation
The mechanism of benzaldehyde condensation involves the following steps:
- Deprotonation: The base catalyst deprotonates the α-carbon of the aldehyde molecule, forming an enolate anion.
- Addition: The enolate anion attacks the carbonyl carbon of another aldehyde or ketone molecule, forming a tetrahedral intermediate.
- Elimination: A proton is transferred from the tetrahedral intermediate to the base catalyst, forming the α-or β-hydroxy ketone product.

4. Factors Affecting the Yield of Benzaldehyde Condensation
There are a number of factors that can affect the yield of benzaldehyde condensation, including:
- Nature of the aldehyde: Aromatic aldehydes generally react more readily in benzaldehyde condensation than aliphatic aldehydes.
- Strength of the base catalyst: Strong bases, such as alcoholate, sodium hydroxide and potassium hydroxide, are more effective catalysts for benzaldehyde condensation than weak bases.
- Reaction temperature: The reaction rate of benzaldehyde condensation increases with temperature. However, high temperatures can also lead to side reactions and product degradation.
- Reaction time: Benzaldehyde condensation typically requires several hours to complete. However, the reaction time can be reduced by using a more concentrated base catalyst or by increasing the reaction temperature.
5. Examples of Benzaldehyde Condensation Reactions
Benzaldehyde condensation can be used to synthesize a wide range of organic compounds, including: benzoin, cinnamic acid, cinnamaldehyde and benzalacetone,
5.1. Benzoin Condensation

Benzoin is a symmetric α-hydroxy ketone that is produced by the benzoin condensation of two benzaldehyde molecules. Benzoin is used as an intermediate in the synthesis of a number of other compounds, such as hydrobenzoin, benzoin isoxazole, and benzoin ethyl ether.
The benzoin condensation was first reported by Justus von Liebig and Friedrich Wöhler in 1832. They discovered that when benzaldehyde was treated with potassium cyanide, a new compound was formed, which they named “benzoin”.
Later, Nikolay Zinin developed a catalytic version of this reaction. He found that by using a small amount of cyanide, the reaction could be carried out more efficiently. This marked a significant advancement in the field of organic chemistry.
5.1.1. Reaction Mechanism
The mechanism of benzaldehyde condensation involves several steps. First, a nucleophile, such as cyanide or N-heterocyclic carbene, attacks the carbonyl carbon of benzaldehyde. This forms an intermediate complex, which then undergoes proton transfer to form benzoin.
In benzaldehyde condensation, nucleophiles play an important role. They attack the carbonyl carbon of benzaldehyde, initiating the reaction. Common nucleophiles used in this reaction include cyanide and N-heterocyclic carbene.
5.2. Benzaldehyde condensation with acetaldehyde

Benzaldehyde condensation with acetaldehyde is a chemical reaction that produces cinnamaldehyde, a fragrant compound used in perfumes and food flavorings. The reaction is also used to synthesize other important chemicals, such as cinnamic acid and cinnamaldehyde derivatives.
5.2.1. Mechanism of the reaction
This reaction is a classic example of a crossed aldol condensation, in which two different aldehydes react to form a new carbon-carbon bond. The reaction proceeds through the following steps:
- Deprotonation of acetaldehyde: A strong base, such as sodium hydroxide, deprotonates acetaldehyde to form the enolate ion.
- Attack on the carbonyl carbon of benzaldehyde: The enolate ion attacks the carbonyl carbon of benzaldehyde, forming a new carbon-carbon bond.
- Dehydration: The resulting alkoxide ion is protonated and then dehydrates to form cinnamaldehyde.
5.2.2. Conditions for the reaction
The reaction is typically carried out in a solvent such as ethanol or water, in the presence of a strong base such as sodium hydroxide. The reaction temperature can range from room temperature to reflux, depending on the desired yield and purity of the product.
One potential side reaction of the reaction is the self-condensation of acetaldehyde to form aldol. To minimize this side reaction, it is important to add the acetaldehyde slowly to the reaction mixture and to keep the reaction temperature low.
5.3. Condensation of Benzaldehyde with Acetic Anhydride (Perkin Condensation)

The condensation of benzaldehyde with acetic anhydride to form cinnamic acid is a classic organic synthesis reaction known as the Perkin condensation. It is a versatile and efficient method for preparing α,β-unsaturated carboxylic acids, which are important intermediates in the synthesis of many natural and synthetic products.
5.3.1. Reaction Mechanism
The Perkin condensation is a base-catalyzed reaction. The sodium acetate catalyst deprotonates the α-carbon of the acetic anhydride molecule, forming a carbanion. This carbanion then attacks the carbonyl carbon of the benzaldehyde molecule, forming a tetrahedral intermediate. The intermediate then collapses, eliminating acetic acid and forming cinnamic acid.
The Perkin condensation is typically carried out at temperatures of 160-180°C. The reaction can be catalyzed by a variety of bases, including sodium acetate, sodium hydroxide, and pyridine.
5.4. Benzaldehyde Condensation with Acetone
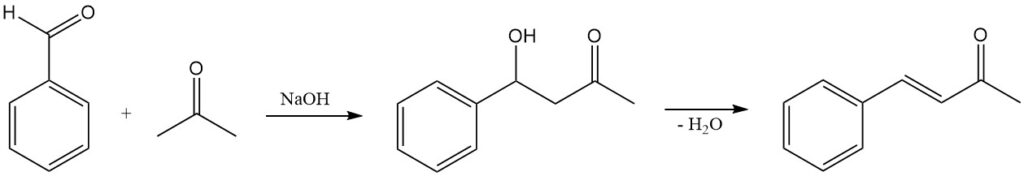
Benzaldehyde condensation with acetone is a common organic reaction used to synthesize benzalacetone, a valuable intermediate in the production of pharmaceuticals, dyes, and other chemicals. The reaction is typically catalyzed by a base, such as sodium hydroxide or potassium hydroxide, and proceeds by a classic aldol condensation mechanism.
5.4.1. Mechanism
The first step in the reaction is the deprotonation of acetone by the base to form the enolate ion. The enolate ion is a strong nucleophile and attacks the electrophilic carbonyl carbon of benzaldehyde. The resulting tetrahedral intermediate collapses, expelling the hydroxide ion and forming a new carbon-carbon bond.
The second step in the reaction is the dehydration of the aldol product to form the α,β-unsaturated ketone benzalacetone. The dehydration step can be promoted by heating the reaction mixture or by adding an acid catalyst.
5.4.2. Production process
The aldol condensation of benzaldehyde and acetone can be carried out in a variety of solvents, but water is the most common. The reaction is typically heated to reflux (around 100 °C) to promote the dehydration step.
The amount of base used depends on the desired yield and purity of the product. For a high yield of pure benzalacetone, it is important to use a slight excess of base.
After the reaction is complete, the product can be isolated by cooling the reaction mixture and extracting it with an organic solvent, such as dichloromethane or diethyl ether. The organic extract can then be washed with water and dried over sodium sulfate or magnesium sulfate.
The crude product can be purified by recrystallization from ethanol or hexanes.
6. Applications of Benzaldehyde Condensation
Benzaldehyde condensation has a wide range of applications in the pharmaceutical, food, and materials industries. Some specific examples include:
- Pharmaceuticals: Benzaldehyde condensation is used to synthesize a variety of important pharmaceuticals, such as phenytoin (anti-seizure medication).
- Food: Benzaldehyde condensation is used to synthesize a number of food additives, such as cinnamaldehyde and coumarin (flavoring and fragrance agents), cinnamic acid (food preservative)
- Organic intermediaires: Benzaldehyde condensation is used to synthesize a variety of raws materials for organic synthesis like benzalacetone, benzil.
References
- Benzoin Condensation: Definition, Mechanism and Applications – https://testbook.com/chemistry/benzoin-condensation
- Carbonyl Condensation Reactions (Summary) – https://www.organicchemistrytutor.com/topic/benzoin-condensation/
- Benzoin Condensation – an overview. – https://www.sciencedirect.com/topics/chemistry/benzaldehyde
- https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/chem_background/exsumpdf/cinnamaldehyde_508.pdf
- SYNTHESIS OF CINNAMIC ACID BASED ON PERKIN REACTION USING SONOCHEMICAL METHOD AND ITS POTENTIAL AS PHOTOPROTECTIVE AGENT
- https://www.researchgate.net/publication/43656185_Synthesis_of_Hydroxyl_Radical_Scavengers_from_Benzalacetone_and_its_Derivatives
FAQ
The two main types of benzaldehyde condensation are the benzoin condensation and the aldol condensation.
The mechanism of benzaldehyde condensation involves the deprotonation of the α-carbon of the aldehyde molecule, followed by an addition reaction with another aldehyde molecule and a final elimination reaction to form the α,β-unsaturated ketone product.
When benzaldehyde is condensed with acetone, a (-C=C-) bond formation occurs. This reaction is an example of a mixed aldol condensation reaction, which results in the formation of benzalacetone.
