Ammonium Sulfate: Properties, Production and Uses
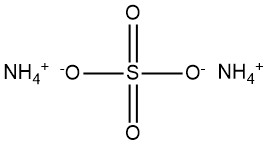
Ammonium sulfate is a chemical compound with the formula (NH4)2SO4. It is commonly encountered as a white, crystalline powder or granular substance. It has emerged as a significant compound during the 19th century produced from ammonia found in coke-oven gas.
As the 20th century commenced, the industrial synthesis of ammonia gained prominence leading to the adoption of ammonium sulfate as a fertilizing agent.
Over time, however, its significance has waned due to the advent of more potent nitrogen-based fertilizers. Notably, its application has persisted on a smaller scale for specific industrial applications.
Since approximately 1960, ammonium sulfate has experienced growing production as a co-product in the organic synthesis procedures. In certain regions, ammonium sulfate is produced from gypsum.
Table of Contents
1. Properties of Ammonium Sulfate
Ammonium sulfate, having a molar mass of 132.14 g/mol, displays a density of 1.77 g/cm³ at 20 °C, along with an average specific heat capacity of 1.423 J/g·K within the temperature range of 2 to 55 °C.
Its crystal structure is rhombic bipyramidal. It’s noteworthy that the size and form of the crystals can be influenced by substances present in the crystallizing solution, and this aspect is significant in the commercial production.
The thermal behavior of ammonium sulfate is intricate. At atmospheric pressure, it cannot be melted without undergoing decomposition, leading to the release of ammonia and the formation of bisulfate.
Yet, in the case of pure anhydrous ammonium sulfate, the vapor pressure of ammonia remains practically zero until 80 °C is reached. Upon surpassing 300 °C, decomposition results in the generation of N2, SO2, SO3, and H2O, apart from ammonia.
Ammonium sulfate does not form hydrates. The heat change upon dissolving 1 mol of the salt in 400 mol of water is +9.92 kJ at 18 °C, while the integral heat of solution stands at +6.57 kJ/mol at 30 °C. The differential heat of solution for a saturated solution is +6.07 kJ/mol at 30 °C.
Ammonium sulfate exhibits deliquescence only above 80% relative humidity, enabling its storage in dry air and ammonia addition significantly reduces its solubility.
At 10 °C, the solubility declines from 73 g (NH4)2SO4 in 100 g of water to approximately 18 g of salt in 100 g of a 24.5% aqueous ammonia solution.
The solubility of calcium sulfate in ammonium sulfate solution is approximately double compared to its solubility in water. This property supports the reaction of gypsum or anhydrite with solutions containing ammonium carbonate – ammonium sulfate.
The compound remains insoluble in typical organic solvents. Dissolution of ammonium sulfate in ethanol, propanol, butanol, acetone, pyridine, and similar solvents results in the formation of two distinct phases: an aqueous phase and an organic solvent phase.
Ammonium sulfate solutions and its solutions in sulfuric acid do not corrode special Cr-Ni stainless steel. Iron and aluminum remain unreactive in ammoniacal solutions. In cases where the solutions contain corrosive substances for stainless steel (e.g., Cl–), vessels can be safeguarded by lining them with acid-resistant bricks.
2. Production of Ammonium sulfate
Ammonium sulfate is produced industrially from:
- Coke-oven gas
- Ammonia and sulfuric acid
- Organic syntheses, such as the production of caprolactam
- Gypsum, ammonia, and carbon dioxide
2.1. From Coke-Oven Gas
The utilization of coke-oven gas as a precursor for ammonium sulfate production has experienced a substantial decline in recent decades. This trend can be attributed to the partial closure of steel mills and advancements in coking methods that yield reduced quantities of ammonium sulfate.
In the direct method, untreated coke-oven gas is introduced into sulfuric acid, resulting in ammonium sulfate tainted by pigmented tar derivatives.
Alternatively, the indirect process involves the extraction of ammonia from coke-oven gas by water washing, followed by liberation using a lime suspension, and combining it with sulfuric acid.
2.2. From Ammonia and Sulfuric Acid
The heat generated from the reaction between ammonia and sulfuric acid can effectively vaporize water if the acid concentration exceeds 70%. The reaction is represented as follows:
2 NH3(g) + H2SO4(l) → (NH4)2SO4(s) ΔH = -274 kJ/mol
In contemporary practice, the reaction takes place within saturators, devices derived from earlier-used evaporation crystallizers. The saturator process integrates neutralization and crystallization within a single apparatus.
Sulfuric acid is introduced on the suction side, while ammonia is added on the pressure side of a forced circulation pump. The ensuing metastable solution yields particles ranging from 0.5 to 3 mm in size upon crystallization.
Continuous discharge, centrifugation, drying, and cooling processes facilitate ammonium sulfate isolation. To facilitate crystal growth, small quantities of phosphoric acid, urea, or inorganic salts are added.
2.3. Coproduct in Organic Syntheses
Ammonium sulfate emerges as a byproduct during the synthesis of synthetic fiber intermediates, such as caprolactam, acrylonitrile, and methyl methacrylate, along with the production of formic acid and acrylamide.
The important source is caprolactam production, vital for nylon 6 synthesis. Traditional caprolactam processes yield 2.5 – 4.5 tons of ammonium sulfate per ton of lactam. Recent processes developed by various entities have succeeded in reducing this range to 1.7 – 1.8 tons per ton of lactam.
2.4. From Gypsum
Both anhydrite and gypsum react with NH3 and CO2:
CaSO4·2 H2O + (NH4)2CO3 → (NH4)2SO4 + CaCO3·2 H2O
This process, established by BASF during World War I, remains significant in regions with limited access to sulfuric acid, including India, Pakistan, and Turkey. Finely ground gypsum is exposed to ammonium carbonate solution in a cascade of stirred vessels.
The reaction mixture, comprising calcium carbonate and ammonium sulfate solution, is filtrated using rotary vacuum filters. The resulting washed calcium carbonate is used for fertilizing lime, the production of calcium ammonium nitrate, raw material for glass, or filler for rubber or PVC.
The slightly turbid ammonium sulfate solution undergoes filtration using filter presses, followed by acidification with H2SO4 and processing through multi-stage evaporation crystallizers to yield coarse-grained ammonium sulfate.
An alternate technique, the Continental Engineering Process, directly introduces NH3 and CO2 into a gypsum slurry within a tall, cylindrical stirred vessel.
2.5. Other Processes
Several methods using SO2 and atmospheric oxygen have became obsolete.
There is a growing interest in processes employing ammonia to eliminate SO2 from power-station exhaust, as an exemple the Walther process which utilizes a two-stage washing of dust-free exhaust to generate a concentrated ammonium sulfite solution. This solution is subsequently oxidized with atmospheric oxygen and spray-dried into ammonium sulfate powder, which is later granulated.
3. Uses of Ammonium sulfate
The predominant application of ammonium sulfate is as a fertilizer, with minimal industrial usage. In industrialized nations, ammonium sulfate typically arises as a co-product or byproduct and primarily sold as a fertilizer especially in developing regions.
Its limited nitrogen content in industrialized nations contributes to higher transportation costs per unit of nitrogen compared to other nitrogen-based fertilizers.
In Africa and Asia, ammonium sulfate has an important function in fertilizing rice, tea, and rubber crops. However, in regions such as Europe, the United States, and Brazil, it frequently forms a constituent of blended and complex fertilizers.
In the industry, (NH4)2SO4 is used for the synthesis of persulfates, flame-retardant agents, and fire-extinguishing powders.
It demonstrates utility in tanning processes, as well as in industries such as photography, textiles, and glass production. Also, ammonium sulfate serves as a nutrient source for yeast and bacterial cultures.
References
- Ammonium Compounds; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_243
- Industrial Inorganic Chemistry. – https://www.sciencedirect.com/science/article/abs/pii/B9780128498910000035
FAQ
The chemical formula for ammonium sulfate is (NH4)2SO4.
Ammonium sulfate is primarily used as a fertilizer to supply essential nutrients, particularly nitrogen and sulfur, to plants. Additionally, it serves industrial purposes such as the production of persulfates, flame-retardant agents, and fire-extinguishing powders. It finds applications in tanning, photography, textiles, glass manufacturing, and as a nutrient for yeast and bacterial cultures.
The molar mass of ammonium sulfate is approximately 132.14 grams per mole (g/mol).
Ammonium sulfate can be produced by various processes. One method involves reacting ammonia gas with sulfuric acid to yield (NH4)2SO4. It can also be derived from coke-oven gas or as a co-product in organic syntheses. Another approach entails the reaction of gypsum with ammonia and carbon dioxide.
Ammonium sulfate is generally considered safe for humans when used according to established guidelines and regulations. As a fertilizer, it poses minimal risk when applied correctly to crops.
