Industrial Production of Cresols
Since 1965, the synthetic production of cresols has largely increased, superseding recovery from coal tar and refinery caustics due to rising demand. Cresols are primarily produced by different processes such as alkali fusion of toluenesulfonates, alkaline chlorotoluene hydrolysis, splitting of cymene hydroperoxide, and vapor-phase methylation of phenol.
The first three processes use toluene as a starting material and are extensions of established phenol synthesis methods. However, phenol methylation is a dedicated route for cresol and xylenol production.
Each process generates a distinct cresol isomer distribution, and all are of individual importance.
Table of Contents
1. Production of Cresol by Alkali Fusion of Toluenesulfonates
Alkali fusion is an important industrial method primarily used for the synthesis of p-cresol. This process comprises four distinct reaction steps:
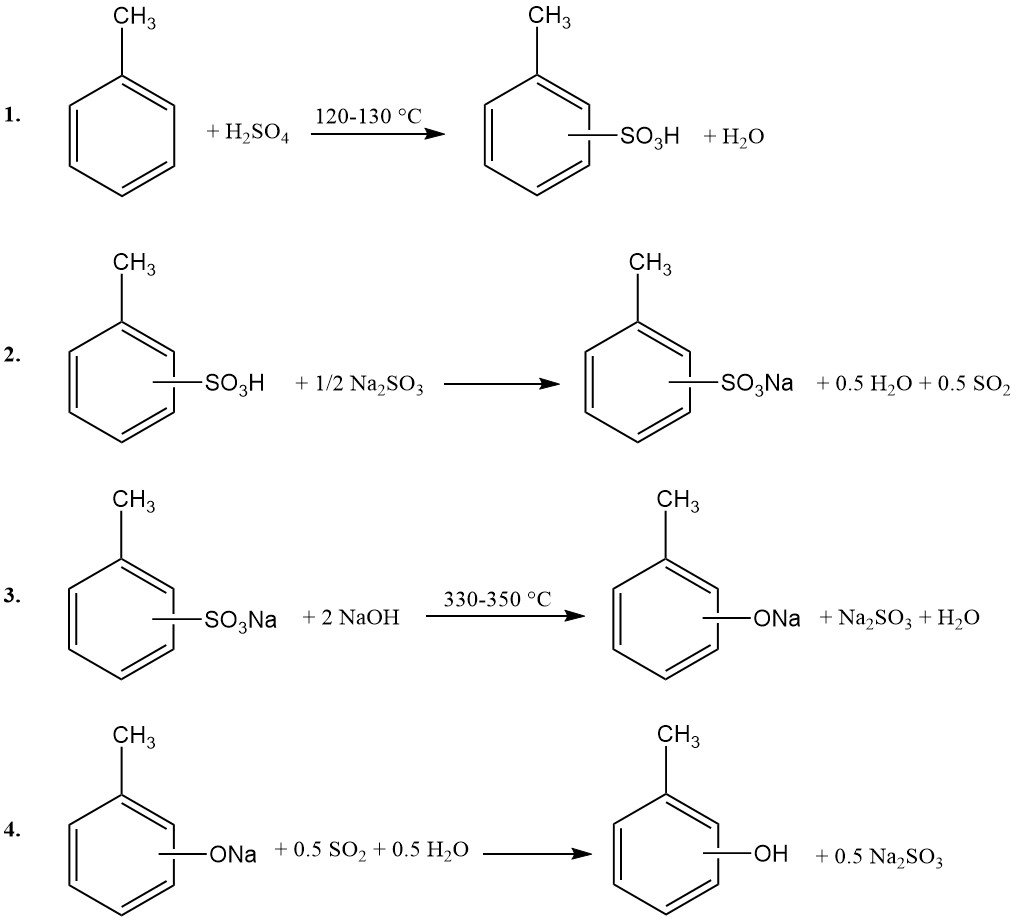
1. Sulfonation of Toluene: Toluene reacts with concentrated sulfuric acid at 120–130 °C under atmospheric pressure. To ensure complete sulfuric acid consumption, reaction-generated water is azeotropically removed with excess toluene vapor.
2. Neutralization: The resulting toluenesulfonic acid mixture is neutralized using sodium sulfite or sodium hydroxide.
3. Alkali Fusion: The sodium toluenesulfonate is subsequently fused with excess sodium hydroxide at 330–350 °C.
4. Acidification: The molten product is dissolved in water, and insoluble sodium sulfite is filtered. The aqueous phase is then acidified with sulfur dioxide (recycled from step 2) or sulfuric acid.
The acidification step yields an aqueous sodium sulfite solution, which is recycled to the neutralization unit, and a crude cresol phase. This crude cresol is dehydrated by azeotropic distillation. The plant and process configuration closely resemble those previously used for phenol synthesis via alkali fusion of sodium benzenesulfonate.
Fractional distillation of the dehydrated crude cresol gives phenol, o-cresol, a m-/p-cresol mixture, and a residue containing ditolyl sulfones, xylenols, and other higher phenols. Ditolyl sulfones are byproducts of sulfonation, while phenols and xylenols are formed in minor quantities during alkali fusion.
The cresol isomer distribution is primarily influenced by sulfonation conditions. Typical compositions include 6–12% o-cresol, 6–12% m-cresol, and 80–85% p-cresol. After o-cresol removal by distillation, p-cresol with approximately 90% purity can be obtained.
Further purification to a m-cresol content of about 1% is achievable through melt crystallization. A cresol yield of 80%, based on toluene, is feasible, though yield decreases with increasing m-cresol content.
Kinetically controlled (mild) sulfonation conditions, coupled with mild alkali fusion, can produce cresol mixtures with reduced m-cresol content. For example, sulfonation with chlorosulfuric acid at 33–45 °C has been reported to yield m-cresol-free product with a 90% yield and an o-/p-cresol ratio of 15:85.
Similarly, toluene sulfonation with sulfur trioxide-sulfur dioxide mixtures at 25–50 °C results in toluenesulfonic acid mixtures practically free of the meta isomer, with an o-/p-isomer ratios as low as 3:97.
Operating in a loop reactor at 0 °C with gaseous SO3 diluted by an inert gas (or under vacuum) can reduce o- and m-isomer content to 0.5%. Sulfonation with oleum or sulfuric acid in a rotating film reactor with short residence times also allows for meta-free sulfonation, primarily yielding p-toluenesulfonic acid.
In contrast, thermodynamically controlled sulfonation (high temperature, extended reaction time, low water content) generates an equilibrium mixture containing approximately 5% o-, 54% meta-, and 41% para-toluenesulfonic acid. Alkali fusion of such mixtures subsequently yields an isomer distribution of roughly 5% o-cresol, 56% m-cresol, and 39% p-cresol.
Technical-grade m-cresol can be produced by reacting steam at 165 °C with a meta-isomer-rich toluenesulfonic acid mixture obtained under thermodynamically controlled conditions (followed by isomerization for several hours at 190–200 °C).
This process selectively hydrolyzes ortho- and para-toluenesulfonic acids back to sulfuric acid and toluene. The liberated toluene is distilled off with steam, leaving m-toluenesulfonic acid of over 90% purity alongside sulfuric acid.
Side reactions during isomerization and hydrolysis can be suppressed by adding 5–10 mol% sodium sulfate to the sulfonation batch. This addition also facilitates the removal of the hydrolysis-formed sulfuric acid as a lower phase at 140–150 °C.
The hydrolysis mixture can be neutralized with concentrated sodium hydroxide solution to form sodium sulfate and sodium m-toluenesulfonate. The resulting sodium m-toluenesulfonate solution is then reacted with a sodium hydroxide melt (containing 10–15% potassium hydroxide) at an initial temperature of 330 °C and then increased to 340 °C for fusion.
Distillation of the crude cresol phase obtained after acidification yields m-cresol with up to 98% purity, at an approximate 65% yield based on reacted toluene.
The toluene sulfonation process for p-cresol is employed by PMC (United States, ca. 18,000 t/a), Inspec Fine Chemicals (United Kingdom, ca. 12,000 t/a), Beraton (Russia, ca. 3,000 t/a), and Konan Chemicals (Japan, ca. 4,000 t/a).
Honshu Chemical Industry Co. (Japan) operates a standby plant (ca. 2,500 t/a) capable of producing either m- or p-cresol. A Gujarat Aromatics plant in India, based on the Honshu process, reportedly produces 3,000–5,000 t/a of p-cresol or m-cresol.
While the toluenesulfonic acid-cresol process requires a relatively simple plant, its primary disadvantage is the unavoidable generation of aqueous sodium sulfite.
2. Production of Cresol by Alkaline Chlorotoluene Hydrolysis
Cresols can be produced industrially by alkaline chlorotoluene hydrolysis to yield an isomer mixture with a high m-cresol content. Bayer AG in Germany, a leading synthetic cresol manufacturer with a capacity exceeding 30,000 t/a, uses this process.
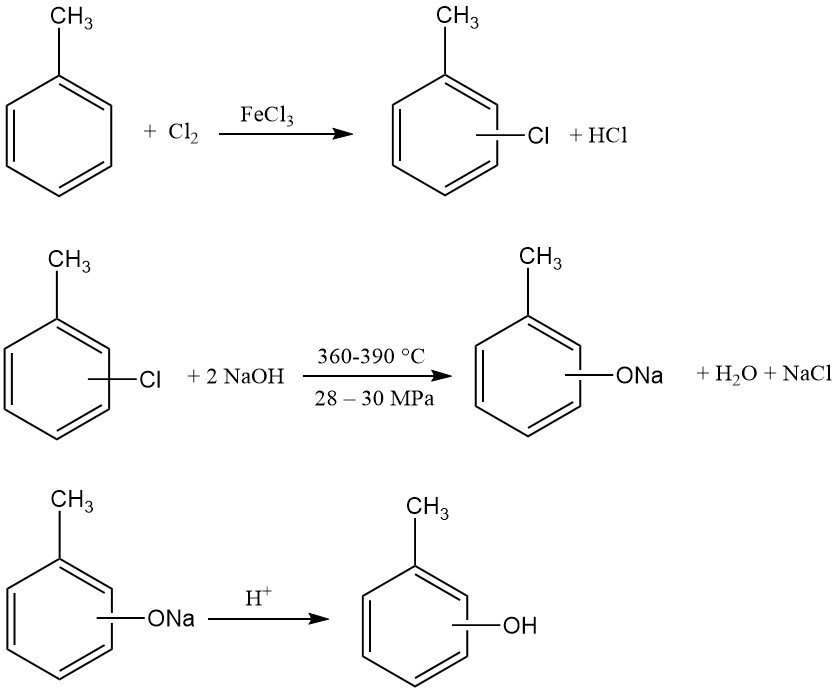
The initial step is the chlorination of toluene. One mole of toluene reacts with one mole of chlorine in the presence of iron(III) chloride and disulfur dichloride, yielding a mixture of o- and p-chlorotoluene. Depending on the FeCl3 and co-catalyst system, o/p ratios can range from 3:1 to 1:2.
Subsequently, this chlorotoluene mixture is hydrolyzed with an excess of sodium hydroxide solution (2.5–3.5 mol/mol chlorotoluene). This reaction occurs at high temperatures (360–390 °C) and pressures (280–300 bar, 28–30 MPa).
The exothermic hydrolysis is conducted continuously in lengthy, high-pressure tubes constructed from nickel steel to resist the corrosive reaction mixture. High flow velocity with frequent changes in direction ensures homogeneity of the reaction mass, preventing component separation.
The resulting sodium cresolate solution is then neutralized to liberate the cresols. The hydrochloric acid generated during the initial chlorination step can be utilized for this neutralization. Co-produced sodium chloride can be recycled to a chlor-alkali electrolysis unit.
Economic efficiency necessitates the co-location of starting material production and the hydrolysis plant, enabling pipeline transport. The underlying technology of this process mostly resemble that of the Dow-Bayer chlorobenzene-to-phenol hydrolysis. However, cresol production generates more byproducts.
These byproducts include ditolyl ethers (bis(methylphenyl) ethers), tolylcresols (methyl(methylphenyl)phenols), and minor amounts of toluene, phenol, benzoic acid, and cracking off-gases (methane, hydrogen). Phenol, for example, can be removed via azeotropic distillation with chlorotoluene.
While tolylcresol formation is difficult to control, ditolyl ether formation can be managed by adjusting temperature, residence time, and sodium hydroxide concentration. Recycling ditolyl ethers also minimizes their accumulation due to their hydrolyzability, albeit at a slower rate than diphenyl ether.
Optimal reaction control allows for cresol yields of approximately 80% based on chlorotoluene.
Since ditolyl ether is a valuable chemical product that is used as a heat transfer medium (e.g., Diphyl DT), an electrical insulator (e.g., Baylectrol 4800, a PCB substitute), and a precursor for tanning agents, it is often not recycled and sometimes process conditions may even be adjusted to enhance its selective formation.
The alkaline chlorotoluene hydrolysis yields high-purity cresols, with minimal other compounds.
A 1:1 o/p-chlorotoluene input typically results in an o-, m-, and p-cresol isomer ratio of approximately 1:2:1. This altered ratio, relative to the chlorotoluene feed, suggests isomerization via aryne intermediates under the severe hydrolysis conditions. After o-cresol distillation, a m-/p-cresol mixture containing approximately 70% m-cresol is obtained.
The plant can also operate with pure o-chlorotoluene or p-chlorotoluene. Hydrolysis of p-chlorotoluene yields a 1:1 m-/p-cresol mixture, while o-chlorotoluene hydrolysis produces a 1:1 o-/m-cresol mixture. Technically, pure m-cresol can be isolated from the latter by distilling off the o-cresol.
Chlorotoluene hydrolysis without significant isomerization is possible under milder conditions (200–350 °C) using alkali metal hydroxides, alkali metal carbonates, ammonium hydroxide, or alkali metal acetate/propionate solutions, in the presence of copper or copper compounds.
These alternative processes are generally not industrially viable due to lower yields at lower temperatures.
3. Production of Cresol by Cymene Hydroperoxide Cleavage
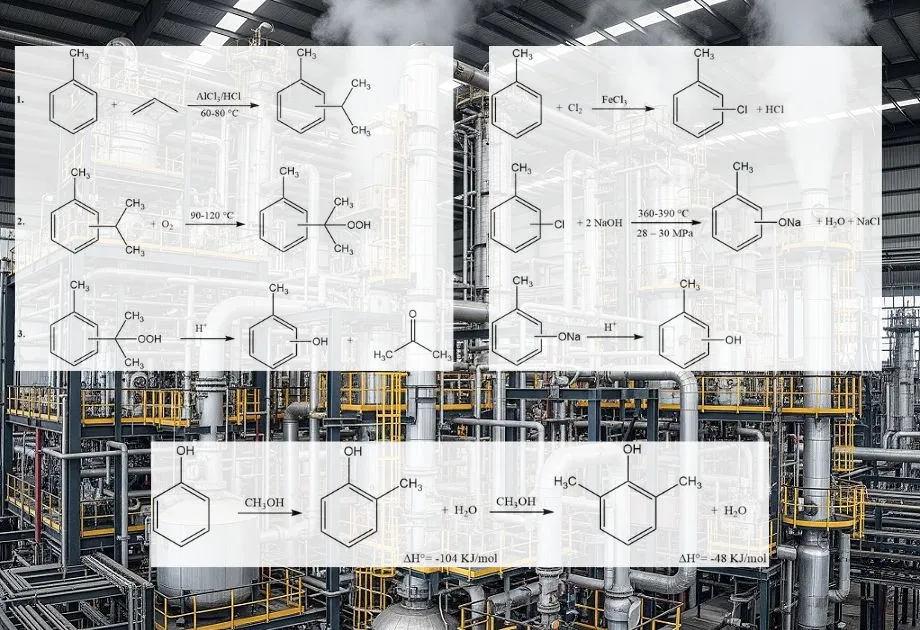
The cymene-cresol process, also known as synthesis via cymene hydroperoxide, enables the production of m- or p-cresol from their corresponding isopropyltoluene (cymene) isomers. This method is not suitable for o-cresol synthesis. The process involves three primary stages:
- Toluene Propylation and Cymene Isomerization: Toluene reacts with propene to form cymene isomers, followed by their isomerization to achieve a desired isomer distribution.
- Cymene Oxidation: Cymene is oxidized to produce cymene hydroperoxide.
- Peroxide Cleavage: The cymene hydroperoxide undergoes acid-catalyzed cleavage to yield cresol and acetone.
The cymene-cresol process presents several operational and economic disadvantages compared to the Hock cumene process. These include lower space-time yields, complex distillation requirements, challenging wastewater management, and reduced product yields (20–30% lower).
Key contributing factors to these limitations are isomer distribution in propylation, suboptimal oxidation kinetics, methyl group oxidation and formaldehyde side reactions, and complex byproduct separation.
1. Toluene propylation yields ortho-, meta-, and para-cymene isomers. Ortho-cymene inhibits the oxidation of other cymenes and is poorly oxidized itself. Maintaining o-cymene content below 10% is critical for continuous oxidation.
Achieving this requires rigorous recycling and isomerization steps, often employing aluminum chloride to establish thermodynamic equilibrium (e.g., 3% o-, 64% m- and 33% p-cymene). Diisopropyltoluene byproducts are also recycled for isomerization.
2. Cymene oxidation rates are slower than cumene, and the maximum achievable peroxide content is lower (ca. 20% vs. >30% for cumene). This necessitates higher recycle ratios of unreacted cymene and leads to increased byproduct formation (e.g., dimethyl(tolyl)methanol, methylacetophenone) at higher oxidation levels.
3. Oxidation of the cymene methyl group is a significant side reaction, producing primary peroxides that are less stable and yield secondary products (e.g., isopropylbenzyl alcohol, cuminic acid). Subsequent acid cleavage generates formaldehyde, which can react with cresol to form resins, substantially reducing cresol yield and complicating product purification.
Techniques to mitigate formaldehyde formation or its effects include selective primary peroxide decomposition/hydrogenation, extraction, or in-situ alkaline dissolution of acidic byproducts.
4. The post-cleavage reaction mixture is highly complex, containing numerous byproducts with similar boiling points and azeotropic tendencies. This makes separation costly. Selective hydrogenation of primary peroxides and subsequent re-hydrogenation of the concentrated cleavage mixture can increase cresol yield by re-forming cymene.
Industrial-scale cymene-cresol plants, using mixed cymene feeds, have been operational since 1969 (e.g., Sumitomo, Mitsui in Japan with 22,000 t/a capacity each), typically producing m-/p-cresol with over 99.5% purity and a 60:40 m/p ratio. High-quality acetone is co-produced.
The use of pure p- or m-cymene feedstock eliminates the need for o-cymene recycling and isomerization, simplifying direct production of pure isomers. Examples include former p-cresol production from natural terpenes (Hercules Powder Co., USA) and current plants using UOP’s Cymex process, which selectively isolates p-cymene using molecular sieves.
4. Production of Cresol by Methylation of Phenol
Synthetic cresols, particularly o-cresol, and xylenols are mainly manufactured by the methylation of phenol with methanol. This process occurs in the presence of various catalysts. 2,6-Xylenol is almost exclusively produced via this method.
This synthesis is a single reaction step. However, it uses phenol, which is a comparatively expensive raw material. Furthermore, separating the products from the reaction mixture is costly because of the close boiling points of several components and the stringent purity requirements for certain products.
The methylation reaction can be done in either the vapor phase or the liquid phase.
4.1. Methylation of Phenol in the Vapor Phase
Vapor-phase methylation of phenol with methanol is a primary industrial route for producing high-purity o-cresol and/or 2,6-xylenol, with the latter almost exclusively manufactured this way.
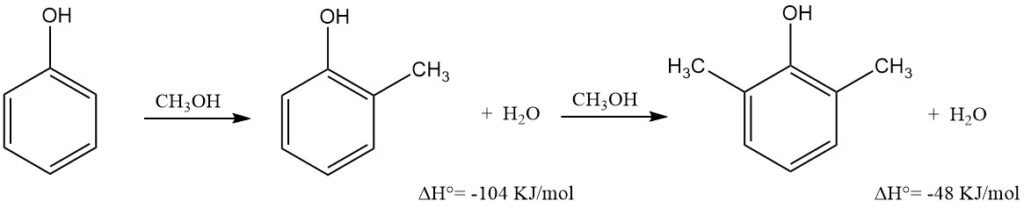
The process involves passing a superheated mixture of phenol, methanol, and water vapors over a fixed metal oxide catalyst in a stainless steel multitubular reactor at 300–460 °C and atmospheric or slightly elevated pressure.
Liquid hourly space velocity (LHSV) typically ranges from 1 to 2 h-1. Reaction temperature is catalyst-dependent and can be adjusted for desired product composition. Reaction heat is managed via boiling organic heat-transfer media, circulating salt melts, or high-pressure steam generation.
Catalyst activity loss over time is counteracted by temperature increases and periodic carbon deposit removal by burning. Water in the feedstock mixture suppresses methanol decomposition, extends regeneration cycles, and prolongs catalyst lifetime.
The reactor effluent exchanges heat with the feedstock and then condenses. Non-condensable methanol decomposition products (H2, CH4, CO2, and CO) are used as fuel gas for steam generation.
Aqueous methanol is distilled from the liquid product and recycled; separation into methanol, phenol-containing water, and phenols can occur in a single distillation column or by decantation with subsequent resin adsorption of phenols.
The remaining water is removed by azeotropic distillation with toluene or as an azeotrope with unreacted phenol, requiring phenol stripping from wastewater in the latter case.
The dehydrated mixture undergoes continuous vacuum fractional distillation in high-efficiency columns to yield phenol ether, phenol, high-purity o-cresol (99.5%), and 2,6-xylenol. The phenol ether–phenol fraction is recycled.
Distillation residue, containing other xylenols and trimethylphenols, is typically used as fuel. If the catalyst exhibits high ortho selectivity, 99.5% pure 2,6-xylenol is directly obtainable. Otherwise, or under severe conditions, the 2,6-xylenol fraction may contain m- and p-cresol, which necessitates further purification.
The o-cresol to 2,6-xylenol ratio is controllable by adjusting the methanol–phenol ratio; recycling o-cresol can make 2,6-xylenol the sole product. Overall yield (selectivity) of o-cresol and 2,6-xylenol, based on reacted phenol, ranges from 90–98%.
The vapor-phase methylation of phenol primarily uses metal oxide catalysts. These catalysts influence reaction temperature, product distribution, and selectivity.
Magnesium oxide catalysts give a high ortho-selectivity and minimize m- and p-cresol formation even at elevated temperatures (420–460 °C). Their performance can be enhanced by combination with other metal oxides (e.g., alkali metals, manganese, copper).
Lower reaction temperatures are achievable with other catalyst systems. Manganese oxide or chromium oxide catalysts operate around 390 °C, while vanadium oxide or iron oxide catalysts are used at 320–350 °C.
These are often mixed with various other oxides (e.g., cerium, aluminum, silicon) to improve conversion and selectivity for 2,6-xylenol/o-cresol. These mixed oxide systems also help extend catalyst lifetime and reduce methanol decomposition.
γ-Aluminum oxide catalyzes the reaction at 300–320 °C with minimal methanol decomposition and good stability. However, it requires significant product recycling due to limited phenol conversion to control byproduct formation and necessitates complex purification steps for 2,6-xylenol.
Catalysts with strong acid sites, such as aluminum oxides, silica-alumina, zeolites, aluminum phosphates, and phosphoric acid-kieselguhr, promote isomerization and transmethylation. They typically yield products with higher m/p-cresol content, especially at elevated temperatures.
Shape-selective zeolites (e.g., HKY type) and phosphoric acid-kieselguhr, reacting at 250–300 °C, can show high selectivity for p-cresol, resulting in m-/p-cresol mixtures with approx. 77–85% p-cresol. Modified Pentasil zeolites also exhibit para-selectivity.
Alternative methylating agents include methylbenzenes, dimethyl ether, methylamine, methane, carbon monoxide-hydrogen mixtures, and formaldehyde-hydrogen mixtures.
Vapor-phase methylation is used globally by companies like General Electric Co. (USA, Netherlands), Inspec Fine Chemicals (UK), Chemopetrol (Czech Republic), Nippon Crenol (Japan), GEM Polymers (Japan), Mitsubishi Gas Chemical Company (Japan), and Honshu Chemical Industry Co. (Japan).
4.2. Methylation of Phenol in the Liquid Phase
Liquid-phase methylation offers an alternative to vapor-phase methods for synthesizing cresols and xylenols. This process typically employs oxide catalysts, such as γ-Al2O3, suspended in a phenol-methanol mixture in an autoclave at 300–400 °C, or over a fixed bed in a tube reactor (e.g., 350 °C, 35 bar).
One notable industrial process, used by Chemisches Werk Lowi, reacts phenol with an equimolar amount of methanol at 350–400 °C using aluminum methylate as a catalyst. This method achieves an approx. 80% yield of cresols and xylenols from approx. 60% converted phenol.
While o-cresol is the main product, other isomers and trimethylphenols are also formed, with product ratios adjustable via reaction parameters. The presence of bases increases conversion, while acids favor p-cresol and 2,4-xylenol.
Another significant process, the Biller process (formerly Union Rheinische Kraftstoff AG), used aqueous zinc halide-hydrogen halide solutions as catalysts at milder conditions (200–240 °C, approx. 25 bar). This method yielded up to 98% cresol from converted phenol but produced larger quantities of p-cresol and 2,4-xylenol, with p-cresol almost free of m-cresol under optimized conditions.
This process required highly corrosion-resistant materials due to the catalyst system.
Both liquid-phase processes face challenges, including raw material costs (phenol) and complex product separation due to similar boiling points and stringent purity demands.
4.3. Transmethylation and Isomerization
Transmethylation and isomerization processes enable the conversion of less desired methylphenol byproducts and residues into more valuable cresol isomers, particularly o-cresol and m-/p-cresol mixtures.
These reactions typically occur by adding phenol to methylphenol residues (from phenol methylation or other sources) or to unused xylenols. The process proceeds in either the vapor phase or the liquid phase, at temperatures ranging from 400–500 °C.
Common catalysts used in these reactions include aluminum oxide, chromium oxide-aluminum oxide, silica-alumina, metal oxide-iron oxide and magnesium oxide-tungsten oxide.
For 2,6-xylenol, transmethylation and accompanying isomerization can occur without a catalyst. This non-catalytic conversion can be performed in the vapor phase at 550–600 °C or batchwise in the liquid phase at 420–470 °C. These methods can enhance the overall o-cresol yield from phenol methylation.
Chiyoda Chemical Engineering Construction Co. developed an isomerization-transalkylation process for cresol isomer mixtures. This process involves reacting cresol mixtures with phenol over silica-alumina catalysts at 400 °C under normal pressure.
The resulting mixture can be rectified to isolate pure o-cresol, with all other phenolic components being recycled.
Historically, attempts to isomerize o-cresol to m- and p-cresol (due to fluctuating demand) using catalysts like aluminum chloride, aluminum fluoride, boron trifluoride, aluminum silicates, or aluminum oxide were largely unsatisfactory. These methods often resulted in undesirable side reactions such as resinification and disproportionation to phenol and dimethylphenols.
However, isomerization without substantial disproportionation is achievable using silica-alumina zeolites of the ZSM group. At approximately 400 °C, with o-cresol conversions of 40–50%, these catalysts can achieve m-/p-cresol selectivities of 90% and an m:p ratio of 7:3.
5. Other Cresol Production Processes
Beyond the primary industrial methods, several alternative syntheses for cresols have been explored, often aiming for isomer selectivity. These processes are generally not yet used on an industrial scale.
5.1. Gulf Oxychlorination
This process involves the oxychlorination of toluene with aqueous hydrochloric acid and oxygen, catalyzed by nitric acid and a palladium or copper salt, at approximately 100 °C. It primarily yields o- and p-chlorotoluene with high selectivity.
Subsequent vapor-phase hydrolysis of the chlorotoluene produces cresol, with the liberated hydrochloric acid being recycled. While efficient and offering high conversion and selectivity, this toluene oxychlorination process demands substantial recycling and corrosion-resistant plant materials.
5.2. Oxidative Decarboxylation of Methylbenzoic Acids
Methylbenzoic acids can be decarboxylated to cresols by passing an air-steam mixture through their melt at 200–240 °C, catalyzed by copper and magnesium salts.
Vapor-phase oxidation with oxygen-nitrogen mixtures over mixed catalysts at around 300 °C shows improved results, with meta-methylbenzoic acid selectively yielding m-cresol. This method is more complex than phenol production and faces challenges with tar formation and byproduct generation.
5.3. Baeyer-Villiger Oxidation of p- or o-Methylbenzaldehyde
This route specifically produces p-cresol from p-methylbenzaldehyde (or o-cresol from o-methylbenzaldehyde). It involves the reaction of methylbenzaldehyde with performic acid (generated from hydrogen peroxide and formic acid) to form a tolyl formate, which is then hydrolyzed to the cresol and formic acid. The process offers high yields, with formic acid largely recycled.
5.4. Nucleus Hydroxylation of Toluene
This involves the direct hydroxylation of toluene (or xylene) using various oxidants (e.g., oxygen, hydrogen peroxide, organic peroxides) and catalyst systems (e.g., Friedel-Crafts catalysts, transition metal compounds, microorganisms).
While extensively investigated, challenges include isolating cresols from dilute solutions and the need for extensive recycling of expensive auxiliaries. For instance, specific microorganisms can regioselectively produce p-cresol, and certain systems using hydrogen peroxide with strong acids or titanium-modified zeolites yield o- and p-cresol mixtures.
5.5. Oxidative Methylation of Toluene
This process involves the adiabatic oxidation of toluene with oxygen, methane, and steam at high temperatures (700–750 °C) in a tube reactor. It produces a mixture of aromatics including ethylbenzene, styrene, benzene, phenol, and cresol, suggesting potential for cresol production at petrochemical scales.
5.6. Hock Reaction of Toluene Derivatives
Similar to the cymene-cresol process, other toluene derivatives can undergo peroxidation and cleavage to yield cresol and a corresponding carbonyl compound. Examples include ethylidenebis(p-methylbenzene) and 4-methyl-cyclohexylbenzene, which can be tailored to produce p-cresol.
Oxidation of xylene in the presence of acetic acid can also yield cresyl acetate, which is then hydrolyzed to cresol.
5.7. Hydrogenation of N,N’-Dialkylaminomethylphenols
This method involves the reaction of phenol with paraformaldehyde and a secondary amine (e.g., piperidine) to form aminomethylphenols, which are then hydrogenated over a palladium-carbon catalyst. This typically yields o-cresol and p-cresol in a 1:2 molar ratio, with high yields based on converted phenol.
5.8. Diels-Alder Ring Closure of Isoprene and Vinyl Acetate
This three-step synthetic route selectively produces p-cresol. Isoprene reacts with vinyl acetate in a Diels-Alder cycloaddition to form 1-methylcyclohexen-4-yl acetate, which is subsequently saponified. The resulting 1-methylcyclohexen-4-ol is then catalytically dehydrogenated to p-cresol.
References
- Cresols and Xylenols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a08_025
- Methylation of phenols. – https://patents.google.com/patent/US3446856A/en
- Synthesis method of o-cresol and m-cresol. – https://patents.google.com/patent/CN103992210A/en
