Bromine: Reactions, Production and Uses

Bromine is a liquid that possesses high density, dark red coloration, and strong fuming properties, in addition to being highly corrosive and lacrimatory. The odor of bromine is so pungent that it can be detected at a volume of 1 ppm in air.
The color of bromine is temperature-dependent, with yellow-orange hues at 20 K, progressing to orange-red, red-brown, and eventually almost black at its melting point. Liquid bromine maintains a dark red hue, while its vapors are generally orange to red-brown.
A. J. Balard discovered bromine in 1824 while studying flora in a salt marsh near Montpellier, France. During his research, he observed a deposit of sodium sulfate that had crystallized in a pan of mother liquor derived from common salt.
While investigating potential uses for the waste liquors, BALARD discovered that the saturation of chlorine could produce a new red liquid that could be distilled.
Table of Contents
1. Chemical Reactions of Bromine
The robust oxidizing properties of bromine are responsible for many of its chemical reactions. The electron affinity of bromine is intermediate between those of chlorine and iodine, as are most of its other chemical properties.
Bromine acts as a moderately strong oxidizing agent and is oxidized by chlorine to produce elemental bromine in keeping with its position in the electromotive series.
Bromine is reduced to bromide in water, with the reducing agent being either the water or the remaining bromine. Oxygen is formed in the former case, as shown by the equation:
2 Br2 + 2 H2O → 4 H+ + 4 Br– + O2 E0298 = +0,25 V
Bromine reacts with hydrogen cyanide to form cyanogen bromide:
Br2 + HCN → BrCN + HBr
Carbonates react with bromine, producingg bromide and bromate salts as a result:
3 Br2 + 3 Na2CO3 → 5 NaBr + NaBrO3 + 3 CO2
Bromine can oxidize sulfur dioxide to sulfuric acid, as demonstrated by the equation:
Br2 + SO2 + 2 H2O → 2HBr + H2SO4
Bromine reacts with red phosphorus and other phosphorus compounds to produce phosphoric acid and hydrogen bromide, as following:
3 Br2 + 2 P + 6 H2O → 6 HBr + 2 H3PO3
H3PO3 + Br2 + H2O → H3PO4 + 2 HBr
H3PO3 + Br2 + NaOH → NaH2PO4
Compounds containing nitrogen, for example ammonia, hydrazine, nitrites and azides also undergo oxidation by bromine, elemental nitrogen being the frequent product of these reactions.
At higher temperatures, bromine reacts directly with hydrogen to produce hydrogen bromide, which is the basis for commercial production of HBr using catalysts such as heated charcoal and finely divided platinum metal.
Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. Potassium and cesium react violently with bromine.
Aluminum and titanium are also highly reactive with bromine, with aluminum emitting light upon reaction, while magnesium, silver, nickel, and lead become coated with their bromides, preventing further reaction.
The protective coating of lead makes it a useful material for containers to hold bromine, as do nickel, tantalum, Hastelloy C, and Monel and other copper alloys.
Moisture is a key factor in the corrosion of metals by bromine, likely due to the hydrolysis products, hydrobromic and hypobromous acids.
At moisture contents below 40 ppm, nickel containers can be used for transporting bromine, but mercury should not be used in instruments and gauges exposed to bromine vapor.
Dry bromine reacts slowly with iron to form a protective layer of ferric bromide, but when wet, a mixture of hydrated iron bromides is produced, which does not adhere to the iron surface.
Several other heavy metals, such as copper, manganese, chromium, antimony, cobalt, cadmium, and bismuth, react with bromine, with some metals requiring higher temperatures for reaction to occur.
1.1. Alkene and Alkyne Bromination
Bromine is capable of readily adding to unsaturated compounds. These reactions are typically carried out at low temperatures to prevent substitution side reactions. While a catalyst is not usually required, high temperatures or ultraviolet radiation can speed up the reaction.
Several important commercial products can be obtained through bromination of unsaturated compounds, including ethylene bromide, acetylene tetrabromide, 2,3-dibromopropanol, hexabromocyclodecane, and tetrabromobisphenol A bis(2,3-dibromopropylether).
1.2. Bromination of Aromatic Compounds
Bromination of aromatic compounds can take place through three types of reactions: (1) addition, (2) substitution on side chains, and (3) substitution on the aromatic ring.
The addition of bromine across aromatic double bonds is typically a slow reaction when catalyzed by light, but chlorine can increase the reaction rate. Bromination of aromatic side chains usually proceeds through a free-radical reaction.
Electrophilic substitution on the aromatic ring is the most significant type of aromatic bromination. In the presence of a catalyst, bromine can react with aromatic compounds to produce aryl bromides and hydrogen bromide through the following reaction:
ArH + Br2 → ArBr + HBr
Suitable catalysts for aromatic bromination include Lewis acids like halides of aluminum, iron, zinc, or antimony. Activated aromatic compounds, like phenol, aniline, and aromatic ethers, can be brominated without the use of a catalyst.
Conversely, very strongly deactivated aromatic compounds containing electron-withdrawing groups can undergo bromination in the presence of sulfuric acid with nitric acid.
Bromine generated from hydrogen bromide in situ is more efficiently used in aromatic substitution reactions:
ArH + HBr + Cl2 → ArBr + 2 HCl
Preformed bromine chloride can also be used for this purpose. Substitution reactions that use BrCl are generally much faster than those that use bromine alone.
1.3. Free-Radical Bromination
Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. The reaction proceeds through the following steps:
Br2 → 2 Br•
RH + Br• → R• + HBr
R• + Br2 → RBr + Br•
Initiation of the reaction requires the dissociation of bromine molecules, which can be achieved by thermal, photolytic, or peroxide initiators. Compared to chlorine, the bromine atom is less reactive, and the hydrogen abstraction step is endothermic, resulting in relatively slow reaction rates.
Nevertheless, the bromine atom exhibits high position selectivity. For instance, in the bromination of n-butane, secondary hydrogen atoms are replaced 82 times faster than primary ones. Bromination of aromatic side chains is much faster than that of alkanes.
1.4. Production of Hydrogen Bromide
Hydrogen bromide is an important intermediate in the manufacture of a variety of organic and inorganic bromides, including medicinal products, dyes, perfumes, photographic chemicals, and many other chemical compounds.
The common commercial method of preparing hydrogen bromide involves the direct gas-phase reaction of hydrogen and bromine, which can be accomplished without a catalyst by sustaining a self-sustaining flame within a closed burner.
Br2 + H2 → 2 HBr + Heat
In the laboratory, hydrogen bromide can be generated by distilling a solution of sodium or potassium bromide with phosphoric or dilute sulfuric acid (5.8 M), but more concentrated sulfuric acid or allowing the reaction temperature to go above 75 °C is not effective as the initially-formed HBr will oxidize to gaseous bromine (Br2).
Alternatively, hydrogen bromide can be generated through the reduction of bromine with phosphorous acid:
H3PO3 + Br2 + H2O → H3PO4 + 2 HBr
1.5. Bromination Using Bromine Carriers
Bromine complexes and bromoimides are utilized in bromination reactions that require selectivity. N-bromosuccinimide (NBS) is the most commonly used bromine carrier in laboratory brominations.
NBS has a unique application in free-radical allylic brominations:

Other effective carriers of bromine for chemical reactions include dibromodimethylhydantoin, dioxane dibromide, pyridine hydrobromide dibromide, and several types of quaternary ammonium polybromides.
2. Production of Bromine
Bromine is produced from naturally occurring bromine-containing liquors (brines) or from seawater, as well as from the manufacture of potassium salts.
Isolating bromine from these sources is challenging due to the significantly larger molar concentration of chloride ions compared to bromide ions in most brines and ocean water.
A separation method of high selectivity is required to extract bromine. Fortunately, a method based on the greater ease of oxidizing bromide compared to chloride and the volatility of the oxidation product, bromine, exists.
Chlorine is used as the most economical and convenient oxidant.
The four principal stages in bromine production include oxidation of bromide to bromine, stripping of bromine from the aqueous solution, separation of bromine from the vapor, and purification of bromine.
Two general processes using chlorination for bromine recovery are commonly employed, based on the following chemical reaction:
2 Br– + Cl2 → Br2 + 2 Cl–
- The first process, the steaming-out process, is used for brines and waste liquors containing bromine when the bromide concentration in the brine is above 1000 ppm. Steam is employed in this process to condense the bromine directly from the steam.
- The second process, the blowing-out process, is used for seawater and involves the use of air because of the large volumes of stripping gas required. To concentrate the bromine, it is necessary to trap it in an alkaline or reducing solution.
2.1. Steaming-Out Process
The production of bromine from brines that contain Br- in concentrations of 1–5 g/L follows a process first described by KUBIERSCHKY in 1906.
The raw brine is heated in a heat exchanger (a) and then passed through two packed towers (b), (c) by gravity flow (Figure 1). In the upper tower, the brine meets a recycled stream of gases that absorb chlorine and bromine.
Near the bottom of the lower tower (c), chlorine and steam are introduced. As they pass upward, the chlorine reacts with bromide in the brine, producing a mixture of bromine vapor and chlorine (about 85: 15 by weight) with steam that is taken off the top.
The water and most of the halogens are condensed (d), and the liquid phases enter a gravity separator (e), while the gas goes to the upper tower.
From the separator, water containing dissolved halogens is sent to the lower (steaming-out) tower, and the heavier bromine layer, containing some chlorine, flows to a fractionating column (g).
The chlorine vapors join the stream to the upper tower, while the liquid bromine, about 99% pure, is drawn off for direct use in the manufacture of bromine compounds or for further purification.
The hot debrominated brine is treated to neutralize acidity (h) and to reduce free halogens, if necessary, and then passed through the heat exchanger, where it warms the incoming brine.
Recent modifications of the original Kubierschky process are related to the larger scale of modern operations, corrosion-resistant materials of construction, and the procedures and instrumentation required for control.
The processes are controlled by measuring critical points such as pH, oxidation-reduction potentials, flow rates, temperatures, and pressures.
Acidity is generated when chlorine reacts with reducing substances, such as hydrogen sulfide, in the brine. This acidity enhances the efficiency of bromine liberation by preventing hydrolysis to hypobromous acid.
However, the use of sulfuric acid is not recommended if the brine contains calcium or strontium because they may precipitate as sulfates and foul the tower packing, heat-exchange surfaces, or brine disposal wells. Sulfates will form if the brine contains sulfides.
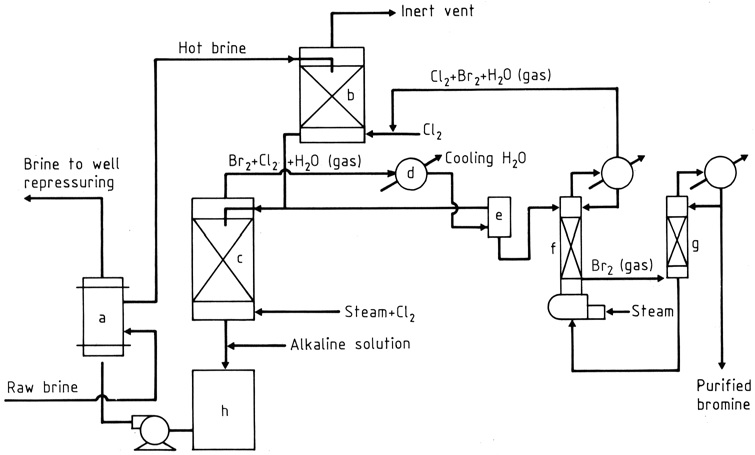
a) Bromine cross heat exchanger; b) Chlorine absorber; c) Steaming-out tower; d) Condenser; e) Separator; f) Stripping column; g) Fractionating column; h) Neutralizer
2.2. Seawater Process
The extraction of bromine directly from seawater, leading to its successful commercial recovery, was first achieved near Wilmington, North Carolina (USA). This was accomplished using an air-blowing method that was initially developed for brine processing by H. H. DOW.
The use of air, rather than steam, was deemed necessary due to the high cost of steam, which would be required to heat the ocean water that contains only about 65 mg/L of bromine. It is noteworthy that the Wilmington plant is no longer operational.
The seawater is pumped to the top of blowing-out towers, with the addition of sulfuric acid and chlorine being performed just above the pumps to ensure proper mixing as it ascends.
A quantity of 1.3 kg of 10% sulfuric acid per ton of water is needed to neutralize the natural hydrogen carbonates and bring the pH to 3.5, and an excess of 15% chlorine over the theoretical requirement is utilized.
Air is drawn up through the towers, which enables the extraction of a mixture of bromine and chlorine (or bromine chloride) from the descending ocean water. The air is subsequently drawn through absorber towers, where it is counter-currently scrubbed using a sodium carbonate solution.
The several reactions that occur may be roughly summarized by the following equation:
3 Na2CO3 + 2 Br2 + BrCl → NaBrO3 + 4 NaBr + NaCl + 3 CO2
To eliminate spray from the air, small packed chambers are installed between the absorber towers and the fans.
When the alkalinity of the scrubber solution is nearly depleted, the solution is transferred to a storage tank and then to a reactor, where it is treated with sulfuric acid and steamed out to release bromine. The chemical reaction is described as follows:
NaBrO3 + 5 NaBr + 3 H2SO4 → 3 Br2 + 3 Na2SO4 + 3 H2O
Other methods have been utilized to extract bromine, including the use of electrolysis in early production in Germany, which was employed instead of chlorine to oxidize bromides.
In the United States, a modified Kubierschky process has been employed to recover bromine as a byproduct from potassium chloride production liquors.
3. Uses of Bromine
Bromine is a versatile element with a wide range of applications. It is used extensively in the production of flame retardants, drilling fluids, organic synthesis, pharmaceuticals, biocides for water treatment, and agriculture.
Additionally, it is used in the manufacturing of dyes, insect repellents, perfumes, and photographic materials.
Other applications of bromine compounds include mercury control and paper manufacturing.
Brominated flame retardants are a significant part of the bromine derivatives. Since the early 1990s, their consumption has risen substantially, and in 2013 they formed more than half of the total bromine consumption.
They are used in industrial and domestic equipment such as computers, furniture, boards, mobile phones, televisions, and textiles.
Bromine compounds’ high-density characteristics are beneficially applied in hydraulic fluids, gage fluids, ore flotation, and drilling fluids.
Calcium, zinc, and sodium bromides are used by the oil and gas drilling industry to prepare high-density, clear brine drilling, completion, packer, and workover fluids.
A significant and expanding group of brominated biocides is for water treatment, although chlorine controls the majority of the water-treatment markets.
In general, both industrial and consumer segments of the water-treatment industry are increasingly replacing chlorine and chlorinated compounds with bromine-based products.
Brominated biocides are also preferred over chlorinated biocides in various industrial applications due to their higher tolerance to a wide range of pH levels, which is a concern in cooling towers and process waters.
Bromine and its compounds are being used to mitigate mercury emissions at coal-fired power plants.
Inorganic bromine compounds such as calcium bromide and sodium bromide bond with mercury present in flue gases from coal-fired power plants creating mercury compounds that are captured in scrubbers, thus removing as much as 90% of the mercury liberated during combustion.
References
- Bromine; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_391.pub2
Bromine; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0218151310010311.a01.pub3
